MicroRNAs, Hypoxia and the Stem-Like State as Contributors to Cancer Aggressiveness
- PMID: 30842790
- PMCID: PMC6391339
- DOI: 10.3389/fgene.2019.00125
MicroRNAs, Hypoxia and the Stem-Like State as Contributors to Cancer Aggressiveness
Abstract
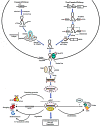
MicroRNAs (miRNAs) are small non-coding RNA molecules that play key regulatory roles in cancer acting as both oncogenes and tumor suppressors. Due to their potential roles in improving cancer prognostic, predictive, diagnostic and therapeutic approaches, they have become an area of intense research focus in recent years. Several studies have demonstrated an altered expression of several miRNAs under hypoxic condition and even shown that the hypoxic microenvironment drives the selection of a more aggressive cancer cell population through cellular adaptations referred as the cancer stem-like cell. These minor fractions of cells are characterized by their self-renewal abilities and their ability to maintain the tumor mass, suggesting their crucial roles in cancer development. This review aims to highlight the interconnected role between miRNAs, hypoxia and the stem-like state in contributing to the cancer aggressiveness as opposed to their independent contributions, and it is based in four aggressive tumors, namely glioblastoma, cervical, prostate, and breast cancers.
Keywords: cancer; cancer aggressiveness; hypoxia; microRNAs; microenvironment; stem-like state.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

