Ni-catalysed reductive arylalkylation of unactivated alkenes
- PMID: 30842845
- PMCID: PMC6369408
- DOI: 10.1039/c8sc04279a
Ni-catalysed reductive arylalkylation of unactivated alkenes
Abstract
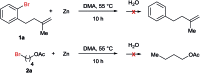
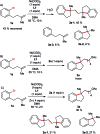
In this protocol Ni-catalysed reductive arylalkylation of unactivated alkenes tethered to aryl bromides with primary alkyl bromides has been accomplished, providing a new path to construct diverse benzene-fused carbo- and heterocyclic cores including indanes, tetrahydroisoquinolines, indolines and isochromanes. Notably, this new method circumvents the pregeneration of organometallics and demonstrates high tolerance to a wide range of functional groups. The preliminary mechanistic investigations suggest a reaction pathway with an intermediate reduction.
Figures

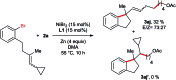


References
-
- For a review on redox-neutral dicarbofunctionalisation of unactivated alkenes, see: KC S., Giri R., J. Org. Chem., 2018, 83 , 3013 . - PubMed
-
-
For selected examples on two-component redox-neutral dicarbofunctionalisation of unactivated alkenes see:
- Phapale V. B., Buñuel E., García-Iglesias M., Cárdenas D. J. Angew. Chem., Int. Ed. 2007;46:8790. - PubMed
- Cong H., Fu G. C. J. Am. Chem. Soc. 2014;136:3788. - PMC - PubMed
- You W., Brown M. K. J. Am. Chem. Soc. 2014;136:14730. - PubMed
- Thapa S., Basnet P., Giri R. J. Am. Chem. Soc. 2017;139:5700. - PubMed
- KC S., Basnet P., Thapa S., Shrestha B., Giri R. J. Org. Chem. 2018;83:2920. - PubMed
-
-
-
For selected examples on three-component redox-neutral dicarbofunctionalisation of unactivated alkenes see:
- Mizutani K., Shinokubo H., Oshima K. Org. Lett. 2003;5:3959. - PubMed
- Terao J., Kato Y., Kambe N. Chem.–Asian J. 2008;3:1472. - PubMed
- Shrestha B., Basnet P., Dhungana R. K., KC S., Thapa S., Sears J. M., Giri R. J. Am. Chem. Soc. 2017;139:10653. - PubMed
- Derosa J., Tran V. T., Boulous M. N., Chen J. S., Engle K. M. J. Am. Chem. Soc. 2017;139:10657. - PubMed
- Derosa J., van der Puy V. A., Tran V. T., Liu M., Engle K. M. Chem. Sci. 2018;9:5278. - PMC - PubMed
- KC S., Dhungana R. K., Shrestha B., Thapa S., Khanal N., Basnet P., Lebrun R. W., Giri R. J. Am. Chem. Soc. 2018;140:9801. - PubMed
- Gao P., Chen L.-A., Brown M. K. J. Am. Chem. Soc. 2018;140:10653. - PMC - PubMed
- Li W., Boon J. W., Zhao Y. Chem. Sci. 2018;9:600. - PMC - PubMed
-
-
- Peng Y., Yan C.-S., Xu X.-B., Wang Y.-W. Chem.–Eur. J. 2012;18:6039. - PubMed
- Peng Y., Xu X.-B., Xiao J., Wang Y.-W. Chem. Commun. 2014;50:472. - PubMed
- Peng Y., Xiao J., Xu X.-B., Duan S.-M., Ren L., Shao Y.-L., Wang Y.-W. Org. Lett. 2016;18:5170. - PubMed
- Kuang Y.-L., Wang X.-F., Anthony D., Diao T.-N. Chem. Commun. 2018;54:2558. - PubMed
- Xiao J., Cong X.-W., Yang G.-Z., Wang Y.-W., Peng Y. Org. Lett. 2018;20:1651. - PubMed
- García-Domínguez A., Li Z., Nevado C. J. Am. Chem. Soc. 2017;139:6835. - PubMed
-
- Very recently, Kong et al. reported Ni-catalysed reductive diarylation of activated alkenes: Wang K., Ding Z., Zhou Z., Kong W., J. Am. Chem. Soc., 2018, 140 , 12364 . - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

