Evaluation of dextromethorphan with select antidepressant therapy for the treatment of depression in the acute care psychiatric setting
- PMID: 30842914
- PMCID: PMC6398352
- DOI: 10.9740/mhc.2019.03.076
Evaluation of dextromethorphan with select antidepressant therapy for the treatment of depression in the acute care psychiatric setting
Abstract
Introduction: Dextromethorphan (DXM), an N-methyl-D-aspartate receptor antagonist, may have ketamine-like antidepressant effects. Dextromethorphan is extensively metabolized via cytochrome P450 (CYP) 2D6, and its half-life in extensive metabolizers is 2 to 4 hours. The purpose of this study was to evaluate the effects of DXM in combination with a moderate-to-strong CYP2D6 inhibitor antidepressant on depression in an acute care psychiatric setting.
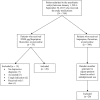
Methods: This was a single-center, retrospective chart review of adult patients with a depressive disorder diagnosis. Patients who received select antidepressant therapy with or without scheduled DXM were included. The primary outcome was the difference in time to improvement of depressive symptoms, which was an average composite of physician documentation, nurse documentation, and first time to 24 hours without as-needed anxiolytics or antipsychotics. The study group consisted of patients who received DXM with select antidepressant therapy, whereas the control group included those who received only select antidepressant therapy.
Results: A total of 40 patients were included. The median time to clinical improvement was 3.00 days and 2.83 days for the study group and control group, respectively (P = .986). The incidence of perceptual disturbances and delusions was higher in the study group as compared with the control group (55% and 35% vs 30% and 25%, respectively).
Discussion: Dextromethorphan was not associated with a rapid antidepressant effect. The commonly used dose of 30 mg daily may have been too low to have an effect; additionally, the most frequently utilized select antidepressant, bupropion, has moderately less CYP2D6 inhibition than fluoxetine and paroxetine.
Keywords: CYP2D6 inhibitor; NMDA; bupropion; depression; dextromethorphan; fluoxetine; paroxetine.
Conflict of interest statement
Disclosures: The authors have nothing to disclose concerning possible financial or personal relationships with commercial entities that may have a direct or indirect interest in the subject matter of this study.
Figures
Similar articles
-
Sex differences in 3,4-methylenedioxymethamphetamine (MDMA; ecstasy)-induced cytochrome P450 2D6 inhibition in humans.Clin Pharmacokinet. 2011 May;50(5):319-29. doi: 10.2165/11584550-000000000-00000. Clin Pharmacokinet. 2011. PMID: 21456632 Clinical Trial.
-
Treatment Resistant Depression with Loss of Antidepressant Response: Rapid-Acting Antidepressant Action of Dextromethorphan, A Possible Treatment Bridging Molecule.Psychopharmacol Bull. 2016 Aug 15;46(2):53-58. Psychopharmacol Bull. 2016. PMID: 27738380 Free PMC article.
-
Assessment of the rapid and sustained antidepressant-like effects of dextromethorphan in mice.Pharmacol Biochem Behav. 2020 Oct;197:173003. doi: 10.1016/j.pbb.2020.173003. Epub 2020 Aug 2. Pharmacol Biochem Behav. 2020. PMID: 32755625
-
Efficacy of dextromethorphan for the treatment of depression: a systematic review of preclinical and clinical trials.Expert Opin Emerg Drugs. 2021 Mar;26(1):63-74. doi: 10.1080/14728214.2021.1898588. Epub 2021 Mar 12. Expert Opin Emerg Drugs. 2021. PMID: 33682569
-
Dextromethorphan/Bupropion: First Approval.CNS Drugs. 2022 Nov;36(11):1229-1238. doi: 10.1007/s40263-022-00968-4. CNS Drugs. 2022. PMID: 36301443 Review.
Cited by
-
Potential Negative Effects of Dextromethorphan as an Add-On Therapy to Methylphenidate in Children With ADHD.Front Psychiatry. 2019 Jun 26;10:437. doi: 10.3389/fpsyt.2019.00437. eCollection 2019. Front Psychiatry. 2019. PMID: 31333511 Free PMC article.
-
Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels.Pharmacol Rev. 2021 Oct;73(4):298-487. doi: 10.1124/pharmrev.120.000131. Pharmacol Rev. 2021. PMID: 34753794 Free PMC article. Review.
-
Exploring the Role of Drug Repurposing in Bridging the Hypoxia-Depression Connection.Membranes (Basel). 2023 Sep 17;13(9):800. doi: 10.3390/membranes13090800. Membranes (Basel). 2023. PMID: 37755222 Free PMC article.
References
-
- Depression [Internet] World Health Organization. Geneva: World Health Organization. 2018 [updated 2018 Mar 22; cited 2018 Nov 12]Available from: http://www.who.int/mediacentre/factsheets/fs369/en/
-
- American Psychiatric Association (APA) [Internet] Arlington (VA): Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. APA; 2010 [cited 2018 Nov 12]. Available from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/g....
-
- Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR. Dextromethorphan: an update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther. 2016;159:1–22. doi: 10.1016/j.pharmthera.2016.01.016. 26826604 DOI: 10.1016/j.pharmthera.2016.01.016 PubMed PMID: PubMed PMID: 26826604. - DOI - PubMed
-
- Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519–30. doi: 10.1176/ajp.2006.163.9.1519. 16946176 quiz 1665. DOI: 10.1176/ajp.2006.163.9.1519 PubMed PMID: PubMed PMID: 16946176. - DOI - PubMed
-
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. 16894061 DOI: 10.1001/archpsyc.63.8.856 PubMed PMID: PubMed PMID: 16894061. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources