Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial
- PMID: 30843046
- PMCID: PMC6938982
- DOI: 10.1093/cid/ciz177
Immunogenicity and Safety of the Adjuvanted Recombinant Zoster Vaccine in Chronically Immunosuppressed Adults Following Renal Transplant: A Phase 3, Randomized Clinical Trial
Abstract
Background: The incidence of herpes zoster is up to 9 times higher in immunosuppressed solid organ transplant recipients than in the general population. We investigated the immunogenicity and safety of an adjuvanted recombinant zoster vaccine (RZV) in renal transplant (RT) recipients ≥18 years of age receiving daily immunosuppressive therapy.
Methods: In this phase 3, randomized (1:1), observer-blind, multicenter trial, RT recipients were enrolled and received 2 doses of RZV or placebo 1-2 months (M) apart 4-18M posttransplant. Anti-glycoprotein E (gE) antibody concentrations, gE-specific CD4 T-cell frequencies, and vaccine response rates were assessed at 1M post-dose 1, and 1M and 12M post-dose 2. Solicited and unsolicited adverse events (AEs) were recorded for 7 and 30 days after each dose, respectively. Solicited general symptoms and unsolicited AEs were also collected 7 days before first vaccination. Serious AEs (including biopsy-proven allograft rejections) and potential immune-mediated diseases (pIMDs) were recorded up to 12M post-dose 2.
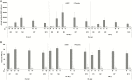
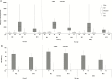
Results: Two hundred sixty-four participants (RZV: 132; placebo: 132) were enrolled between March 2014 and April 2017. gE-specific humoral and cell-mediated immune responses were higher in RZV than placebo recipients across postvaccination time points and persisted above prevaccination baseline 12M post-dose 2. Local AEs were reported more frequently by RZV than placebo recipients. Overall occurrences of renal function changes, rejections, unsolicited AEs, serious AEs, and pIMDs were similar between groups.
Conclusions: RZV was immunogenic in chronically immunosuppressed RT recipients. Immunogenicity persisted through 12M postvaccination. No safety concerns arose.
Clinical trials registration: NCT02058589.
Keywords: herpes zoster vaccine; immunogenicity; immunosuppression; renal transplant; safety.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



Comment in
-
Vaccine Prevention of Herpes Zoster in Organ Transplant Recipients: A Busy Intersection of Immune Responses to Foreign Antigens.Clin Infect Dis. 2020 Jan 2;70(2):191-192. doi: 10.1093/cid/ciz179. Clin Infect Dis. 2020. PMID: 30843045 No abstract available.
-
Letter to the Editor.Clin Infect Dis. 2020 Feb 3;70(4):719. doi: 10.1093/cid/ciz495. Clin Infect Dis. 2020. PMID: 31340381 No abstract available.
-
Reply to Cheng et al and Nellore et al.Clin Infect Dis. 2020 Feb 3;70(4):720-721. doi: 10.1093/cid/ciz496. Clin Infect Dis. 2020. PMID: 31504292 No abstract available.
-
Herpes Zoster Subunit Vaccination for Renal Transplant Recipients.Clin Infect Dis. 2020 Feb 3;70(4):718-719. doi: 10.1093/cid/ciz494. Clin Infect Dis. 2020. PMID: 31504313 No abstract available.
References
-
- Dworkin RH. Inadequate evidence for a revised definition of postherpetic neuralgia (PHN). Pain 2007; 128:189–90. - PubMed
-
- Arvin AM. Immune responses to varicella-zoster virus. Infect Dis Clin North Am 1996; 10:529–70. - PubMed
-
- Gourishankar S, McDermid JC, Jhangri GS, Preiksaitis JK. Herpes zoster infection following solid organ transplantation: incidence, risk factors and outcomes in the current immunosuppressive era. Am J Transplant 2004; 4:108–15. - PubMed
-
- Arness T, Pedersen R, Dierkhising R, Kremers W, Patel R. Varicella zoster virus–associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis 2008; 10:260–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

