Optimised Synthesis of the Bacterial Magic Spot (p)ppGpp Chemosensor PyDPA
- PMID: 30843657
- PMCID: PMC6618120
- DOI: 10.1002/cbic.201900013
Optimised Synthesis of the Bacterial Magic Spot (p)ppGpp Chemosensor PyDPA
Abstract
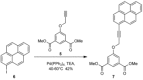
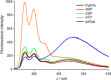
Guanosine penta- or tetraphosphate (pppGpp or ppGpp, respectively) is a nucleotide signalling molecule with a marked effect on bacterial physiology during stress. Its accumulation slows down cell metabolism and replication, supposedly leading to the formation of the antibiotic-tolerant persister phenotype. A specifically tailored fluorescent chemosensor, PyDPA, allows the detection of (p)ppGpp in solution with high selectivity, relative to that of other nucleotides. Herein, an optimised synthetic approach is presented that improves the overall yield from 9 to 67 % over 7 steps. The simplicity and robustness of this approach will allow groups investigating the many facets of (p)ppGpp easy access to this probe.
Keywords: fluorescence; guanosine phosphates; nucleotides; sensors; synthesis design.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
Figures

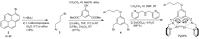


References
-
- Zhou Y., Xu Z., Yoon J., Chem. Soc. Rev. 2011, 40, 2222–2235. - PubMed
-
- WHO, Antimicrobial Resistance: Global Report on Surveillance, 2014; https://www.who.int/drugresistance/documents/surveillancereport/en/.
-
- Potrykus K., Cashel M., Annu. Rev. Microbiol. 2008, 62, 35–51. - PubMed
-
- Cashel M., Gallant J., Nature 1969, 221, 838–841. - PubMed
-
- None

