Spatial Patterns of Brain Activity Preferentially Reflecting Transient Pain and Stimulus Intensity
- PMID: 30844052
- PMCID: PMC6458907
- DOI: 10.1093/cercor/bhz026
Spatial Patterns of Brain Activity Preferentially Reflecting Transient Pain and Stimulus Intensity
Abstract
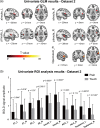
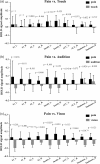
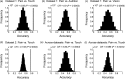
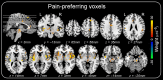
How pain emerges in the human brain remains an unresolved question. Neuroimaging studies have suggested that several brain areas subserve pain perception because their activation correlates with perceived pain intensity. However, painful stimuli are often intense and highly salient; therefore, using both intensity- and saliency-matched control stimuli is crucial to isolate pain-selective brain responses. Here, we used these intensity/saliency-matched painful and non-painful stimuli to test whether pain-selective information can be isolated in the functional magnetic resonance imaging responses elicited by painful stimuli. Using two independent datasets, multivariate pattern analysis was able to isolate features distinguishing the responses triggered by (1) intensity/saliency-matched painful versus non-painful stimuli, and (2) high versus low-intensity/saliency stimuli regardless of whether they elicit pain. This indicates that neural activity in the so-called "pain matrix" is functionally heterogeneous, and part of it carries information related to both painfulness and intensity/saliency. The response features distinguishing these aspects are spatially distributed and cannot be ascribed to specific brain structures.
Keywords: fMRI; multivariate pattern analysis; pain; pain matrix; saliency.
© The Author(s) 2019. Published by Oxford University Press.
Figures

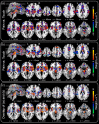
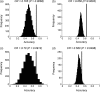
References
-
- Afif A, Minotti L, Kahane P, Hoffmann D. 2010. Anatomofunctional organization of the insular cortex: a study using intracerebral electrical stimulation in epileptic patients. Epilepsia. 51:2305–2315. - PubMed
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 9:463–484. - PubMed
-
- Boly M, Faymonville ME, Schnakers C, Peigneux P, Lambermont B, Phillips C, Lancellotti P, Luxen A, Lamy M, Moonen G, et al. . 2008. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 7:1013–1020. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

