Continuous Glucose Monitoring Predicts Progression to Diabetes in Autoantibody Positive Children
- PMID: 30844073
- PMCID: PMC6589073
- DOI: 10.1210/jc.2018-02196
Continuous Glucose Monitoring Predicts Progression to Diabetes in Autoantibody Positive Children
Abstract
Context: Accurate measures are needed for the prediction and diagnosis of type 1 diabetes (T1D) in at-risk persons.
Objective: The purpose of this study was to explore the value of continuous glucose monitoring (CGM) in predicting T1D onset.
Design and setting: The Diabetes Autoimmunity Study in the Young (DAISY) prospectively follows children at increased risk for development of islet autoantibodies (islet autoantibody positive; Ab+) and T1D.
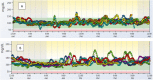
Participants: We analyzed 23 Ab+ participants with available longitudinal CGM data.
Main outcome measure: CGM metrics as glycemic predictors of progression to T1D.
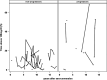
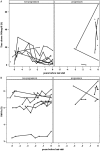
Results: Of 23 Ab+ participants with a baseline CGM, 8 progressed to diabetes at a median age of 13.8 years during a median follow-up of 17.7 years (interquartile range, 14.6 to 22.0 years). Compared with nonprogressors, participants who progressed to diabetes had significantly increased baseline glycemic variability (SD, 29 vs 21 mg/dL; P = 0.047), daytime sensor average (122 vs 106 mg/dL; P = 0.02), and daytime sensor area under the curve (AUC, 470,370 vs 415,465; P = 0.047). They spent 24% of time at >140 mg/dL and 12% at >160 mg/dL compared with, respectively, 8% and 3% for nonprogressors (both P = 0.005). A receiver-operating characteristic curve analysis showed an AUC of 0.85 for percentage of time spent at >140 or 160 mg/dL. The cutoff of 18% time spent at >140 mg/dL had 75% sensitivity, 100% specificity, and a 100% positive predictive value for diabetes prediction, although these values could change because some nonprogressors may develop diabetes with longer follow-up.
Conclusions: Eighteen percent or greater CGM time spent at >140 mg/dL predicts progression to diabetes in Ab+ children.
Copyright © 2019 Endocrine Society.
Figures
References
-
- Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M; DAISY study. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–1404. - PubMed
-
- Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308–313. - PubMed
-
- Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS; Diabetes Prevention Trial-Type 1 Study Group. Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2007;30(1):38–42. - PubMed
-
- Stene LC, Barriga K, Hoffman M, Kean J, Klingensmith G, Norris JM, Erlich HA, Eisenbarth GS, Rewers M. Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes. 2006;7(5):247–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

