Ketamine: A Paradigm Shift for Depression Research and Treatment
- PMID: 30844397
- PMCID: PMC6560624
- DOI: 10.1016/j.neuron.2019.02.005
Ketamine: A Paradigm Shift for Depression Research and Treatment
Abstract
Ketamine is the first exemplar of a rapid-acting antidepressant with efficacy for treatment-resistant symptoms of mood disorders. Its discovery emerged from a reconceptualization of the biology of depression. Neurobiological insights into ketamine efficacy shed new light on the mechanisms underlying antidepressant efficacy.
Published by Elsevier Inc.
Figures
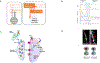
References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, and Krystal JH (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47, 351–354. - PubMed
-
- Fava M, Freeman M, Flynn M, Judge H, Hoeppner B, Cusin C, Ionescu D, Mathew S, Chang L, Iosifescu D, et al. (2017). Double-blind, placebo-controlled trial of ketamine therapy in treatment resistant depression (TRD). In American Society of Clinical Psychopharmacology (Miami, Florida), p. 84 (#W28).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

