Mechanisms of obesity-induced metabolic and vascular dysfunctions
- PMID: 30844720
- PMCID: PMC6689231
- DOI: 10.2741/4758
Mechanisms of obesity-induced metabolic and vascular dysfunctions
Abstract
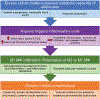
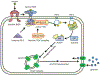
Obesity has reached epidemic proportions and its prevalence is climbing. Obesity is characterized by hypertrophied adipocytes with a dysregulated adipokine secretion profile, increased recruitment of inflammatory cells, and impaired metabolic homeostasis that eventually results in the development of systemic insulin resistance, a phenotype of type 2 diabetes. Nitric oxide synthase (NOS) is an enzyme that converts L-arginine to nitric oxide (NO), which functions to maintain vascular and adipocyte homeostasis. Arginase is a ureohydrolase enzyme that competes with NOS for L-arginine. Arginase activity/expression is upregulated in obesity, which results in diminished bioavailability of NO, impairing both adipocyte and vascular endothelial cell function. Given the emerging role of NO in the regulation of adipocyte physiology and metabolic capacity, this review explores the interplay between arginase and NO, and their effect on the development of metabolic disorders, cardiovascular diseases, and mitochondrial dysfunction in obesity. A comprehensive understanding of the mechanisms involved in the development of obesity-induced metabolic and vascular dysfunction is necessary for the identification of more effective and tailored therapeutic avenues for their prevention and treatment.
Figures
References
-
- Brant LC, Wang N, Ojeda FM, LaValley M, Barreto SM, Benjamin EJ, Mitchell GF, Vasan RS, Palmisano JN and Münzel T: Relations of Metabolically Healthy and Unhealthy Obesity to Digital Vascular Function in Three Community-Based Cohorts: A Meta-Analysis. Journal of the American Heart Association, 6(3), e004199 (2017) DOI: 10.1161/JAHA.116.004199 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

