Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: A general practice-based, observational study
- PMID: 30845156
- PMCID: PMC6405190
- DOI: 10.1371/journal.pone.0213192
Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: A general practice-based, observational study
Abstract
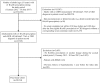
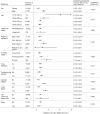
Data on hyperkalemia frequency among chronic kidney disease (CKD) patients receiving renin-angiotensin aldosterone system inhibitors (RAASis) and its impact on subsequent RAASi treatment are limited. This population-based cohort study sought to assess the incidence of clinically significant hyperkalemia among adult CKD patients who were prescribed a RAASi and the proportion of patients with RAASi medication change after experiencing incident hyperkalemia. We conducted a retrospective, population-based cohort study (1 January 2013-30 June 2017) using Australian national general practice data from the NPS MedicineWise's MedicineInsight program. The study included adults aged ≥18 years who received ≥1 RAASi prescription during the study period and had CKD (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73m2). Study outcomes included incident clinically significant hyperkalemia (serum potassium >6 mmol/L or a record of hyperkalemia diagnosis) and among patients who experienced incident hyperkalemia, the proportion who had RAASi medication changes (cessation or dose reduction during the 210-day period after the incident hyperkalemia event). Among 20,184 CKD patients with a median follow-up of 3.9 years, 1,992 (9.9%) patients experienced an episode of hyperkalemia. The overall incidence rate was 3.1 (95% CI: 2.9-3.2) per 100 person-years. Rates progressively increased with worsening eGFR (e.g. 3.5-fold increase in patients with eGFR <15 vs. 45-59 ml/min/1.73m2). Among patients who experienced incident hyperkalemia, 46.6% had changes made to their RAASi treatment regimen following the first occurrence of hyperkalemia (discontinuation: 36.6% and dose reduction: 10.0%). In this analysis of adult RAASi users with CKD, hyperkalemia and subsequent RAASi treatment changes were common. Further assessment of strategies for hyperkalemia management and optimal RAASi use among people with CKD are warranted.
Conflict of interest statement
This study was funded by AstraZeneca Proprietary Limited and commissioned by VentureWise (a wholly owned commercial subsidiary of NPS MedicineWise). MJ reports no conflicts of interest. MJJ is responsible for research projects that have received unrestricted funding from Gambro, Baxter, CSL, Amgen, Eli Lilly, and Merck; has served on advisory boards sponsored by Akebia, Baxter and Boehringer Ingelheim, spoken at scientific meetings sponsored by Janssen, Amgen and Roche; with any consultancy, honoraria or travel support paid to her institution. VP has participated in advisory boards for both Relypsa and AstraZeneca and has received honoraria and consultation fees from Tricida, Novartis, Amgen, Janssen, GlaxoSmithKline, Astellas, Boeringer Ingelheim, Baxter, Mitsubishi Tanabe, Retrophin, Merck, Abbvie, Novo Nordisk, AstraZeneca, Gilead, Durect, Servier, Eli Lilly, Relypsa, Pharmalink, Bayer, Bristol-Myers Squibb and Tufts, with payments paid to his institution. MG reports receiving honoraria from Shire and Amgen for speaking at scientific meetings. QP, LB, AR and KR report no conflicts of interest. Reported competing interests do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous