Pretreatment neutrophil-to-lymphocyte ratio predicts clinical relapse of ulcerative colitis after tacrolimus induction
- PMID: 30845259
- PMCID: PMC6405082
- DOI: 10.1371/journal.pone.0213505
Pretreatment neutrophil-to-lymphocyte ratio predicts clinical relapse of ulcerative colitis after tacrolimus induction
Abstract
Objectives: Although tacrolimus is useful as an induction therapy in patients with ulcerative colitis (UC), information regarding the long-term outcome after tacrolimus therapy is insufficient. The aim of this study was to evaluate the clinical significance of the pretreatment neutrophil-to-lymphocyte ratio (NLR) as a prognostic factor in patients with UC receiving tacrolimus, to aid treatment selection.
Materials and methods: Patients with moderate-to-severe active UC who received oral tacrolimus induction therapy and subsequent immunomodulatory maintenance therapy at our hospital between 2009 and 2017 and who showed clinical response at week 12, were retrospectively enrolled. Cox regression analysis was conducted to study the prognostic role of the pretreatment NLR. The combined impact of the NLR and other known prognostic factors was investigated with multivariate regression.
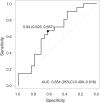
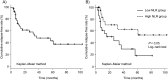
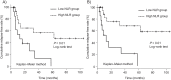
Results: Among 45 patients included in this study, 21 patients experienced relapse during a median follow-up period of 16.6 months. Multivariate Cox regression analysis identified the pretreatment NLR (hazard ratio [HR]: 0.82, 95% confidence interval [CI]: 0.72-0.94, P < 0.01) and the use of immunomodulators at the start of tacrolimus treatment (HR: 0.18, 95% CI: 0.05-0.66, P = 0.01) as independent predictors of clinical relapse.
Conclusions: The pretreatment NLR is an independent prognostic factor in patients with UC treated with tacrolimus.
Conflict of interest statement
The following authors Tetsuya Tanigawa, Toshio Watanabe, and Yasuhiro Fujiwara were previously faculty members of a course sponsored by Eisai Co., Ltd, up to last year. They are now no longer faculty members of this course. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Faubion WA Jr., Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(2):255–60. Epub 2001/08/07. . - PubMed
-
- Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2007;5(1):103–10. Epub 2006/12/05. 10.1016/j.cgh.2006.09.033 . - DOI - PubMed
-
- Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. Journal of Crohn’s & colitis. 2017;11(7):769–84. Epub 2017/05/18. 10.1093/ecco-jcc/jjx009 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

