Hormone replacement therapy attenuates hearing loss: Mechanisms involving estrogen and the IGF-1 pathway
- PMID: 30845368
- PMCID: PMC6516159
- DOI: 10.1111/acel.12939
Hormone replacement therapy attenuates hearing loss: Mechanisms involving estrogen and the IGF-1 pathway
Abstract
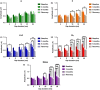
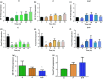
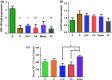
Estradiol (E) is a multitasking hormone that plays a prominent role in the reproductive system, and also contributes to physiological and growth mechanisms throughout the body. Frisina and colleagues have previously demonstrated the beneficial effects of this hormone, with E-treated subjects maintaining low auditory brainstem response (ABR) thresholds relative to control subjects (Proceedings of the National Academy of Sciences of the United States of America, 2006;103:14246; Hearing Research, 2009;252:29). In the present study, we evaluated the functionality of the peripheral and central auditory systems in female CBA/CaJ middle-aged mice during and after long-term hormone replacement therapy (HRT) via electrophysiological and molecular techniques. Surprisingly, there are very few investigations about the side effects of HRT in the auditory system after it has been discontinued. Our results show that the long-term effects of HRT are permanent on ABR thresholds and ABR gap-in-noise (GIN) amplitude levels. E-treated animals had lower thresholds and higher amplitude values compared to other hormone treatment subject groups. Interestingly, progesterone (P)-treated animals had ABR thresholds that increased but amplitude levels that remained relatively the same throughout treatment. These results were consistent with qPCR experiments that displayed high levels of IGF-1R in the stria vascularis (SV) of both E and P animal groups compared to combination treatment (E + P) animals. IGF-1R plays a vital role in mediating anti-apoptotic responses via the PI3K/AKT pathway. Overall, our findings gain insights into the neuro-protective properties of E hormone treatments as well as expand the scientific knowledge base to help women decide whether HRT is the right choice for them.
Keywords: HRT; age-related hearing loss; aging; auditory system; hormone replacement therapy; neurodegeneration.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures



Similar articles
-
Hormone replacement therapy diminishes hearing in peri-menopausal mice.Hear Res. 2009 Jun;252(1-2):29-36. doi: 10.1016/j.heares.2009.02.010. Epub 2009 Mar 6. Hear Res. 2009. PMID: 19269311 Free PMC article.
-
Influence of modified transdermal hormone replacement therapy on the concentrations of hormones, growth factors, and bone mineral density in women with osteopenia.Metabolism. 2009 Jan;58(1):1-7. doi: 10.1016/j.metabol.2008.07.016. Metabolism. 2009. PMID: 19059524 Clinical Trial.
-
Effects of hormonal replacement therapy on plasma sex hormone-binding globulin, androgen and insulin-like growth factor-1 levels in postmenopausal women.J Endocrinol Invest. 1996 Sep;19(8):535-41. doi: 10.1007/BF03349013. J Endocrinol Invest. 1996. PMID: 8905477
-
Hormone Replacement Therapy in Cancer Survivors - Review of the Literature.Pathol Oncol Res. 2020 Jan;26(1):63-78. doi: 10.1007/s12253-018-00569-x. Epub 2019 Jan 8. Pathol Oncol Res. 2020. PMID: 30617760 Free PMC article. Review.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
Cited by
-
Sex-Linked Biology and Gender-Related Research Is Essential to Advancing Hearing Health.Ear Hear. 2023 Jan-Feb 01;44(1):10-27. doi: 10.1097/AUD.0000000000001291. Epub 2022 Nov 17. Ear Hear. 2023. PMID: 36384870 Free PMC article. Review.
-
Age-related hearing loss: An updated and comprehensive review of the interventions.Iran J Basic Med Sci. 2024;27(3):256-269. doi: 10.22038/IJBMS.2023.72863.15849. Iran J Basic Med Sci. 2024. PMID: 38333758 Free PMC article. Review.
-
Loss of Esr1 Does Not Affect Hearing and Balance.bioRxiv [Preprint]. 2024 Mar 6:2024.03.03.583163. doi: 10.1101/2024.03.03.583163. bioRxiv. 2024. PMID: 38496399 Free PMC article. Preprint.
-
The Role of Molecular and Cellular Aging Pathways on Age-Related Hearing Loss.Int J Mol Sci. 2024 Sep 7;25(17):9705. doi: 10.3390/ijms25179705. Int J Mol Sci. 2024. PMID: 39273652 Free PMC article. Review.
-
Sex differences in glutamate AMPA receptor subunits mRNA with fast gating kinetics in the mouse cochlea.Front Syst Neurosci. 2023 Mar 2;17:1100505. doi: 10.3389/fnsys.2023.1100505. eCollection 2023. Front Syst Neurosci. 2023. PMID: 36936507 Free PMC article.
References
-
- Bittar, R. S. M. , Cruz, O. L. M. , Lorenzi, M. C. , Marone, S. A. M. , & Miniti, A. (2001). Morphological and functional study of the cochlea after administration of estrogen and progesterone in guinea pig. International Tinnitus Journal, 7(1), 41–45. - PubMed
-
- Chan, M. , Chow, C. , Hamson, D. K. , Lieblich, S. E. , & Galea, L. A. (2014). Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. Journal of Neuroendocrinology, 26(6), 386–399. 10.1111/jne.12159 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

