EMT Regulation by Autophagy: A New Perspective in Glioblastoma Biology
- PMID: 30845654
- PMCID: PMC6468412
- DOI: 10.3390/cancers11030312
EMT Regulation by Autophagy: A New Perspective in Glioblastoma Biology
Abstract
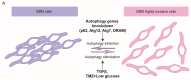
Epithelial-to-mesenchymal transition (EMT) and its reverse process MET naturally occur during development and in tissue repair in vertebrates. EMT is also recognized as the crucial event by which cancer cells acquire an invasive phenotype through the activation of specific transcription factors and signalling pathways. Even though glial cells have a mesenchymal phenotype, an EMT-like process tends to exacerbate it during gliomagenesis and progression to more aggressive stages of the disease. Autophagy is an evolutionary conserved degradative process that cells use in order to maintain a proper homeostasis, and defects in autophagy have been associated to several pathologies including cancer. Besides modulating cell resistance or sensitivity to therapy, autophagy also affects the migration and invasion capabilities of tumor cells. Despite this evidence, few papers are present in literature about the involvement of autophagy in EMT-like processes in glioblastoma (GBM) so far. This review summarizes the current understanding of the interplay between autophagy and EMT in cancer, with special regard to GBM model. As the invasive behaviour is a hallmark of GBM aggressiveness, defining a new link between autophagy and EMT can open a novel scenario for targeting these processes in future therapeutical approaches.
Keywords: Wnt/β-catenin signalling; autophagy; cadherins; epithelial-to-mesenchymal transition (EMT); glioblastoma (GBM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Autophagy induction impairs Wnt/β-catenin signalling through β-catenin relocalisation in glioblastoma cells.Cell Signal. 2019 Jan;53:357-364. doi: 10.1016/j.cellsig.2018.10.017. Epub 2018 Oct 26. Cell Signal. 2019. PMID: 30442596
-
Multifaceted WNT Signaling at the Crossroads Between Epithelial-Mesenchymal Transition and Autophagy in Glioblastoma.Front Oncol. 2020 Nov 12;10:597743. doi: 10.3389/fonc.2020.597743. eCollection 2020. Front Oncol. 2020. PMID: 33312955 Free PMC article. Review.
-
Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells.Mol Oncol. 2015 Oct;9(8):1612-25. doi: 10.1016/j.molonc.2015.04.016. Epub 2015 May 13. Mol Oncol. 2015. PMID: 26022108 Free PMC article.
-
MIR517C inhibits autophagy and the epithelial-to-mesenchymal (-like) transition phenotype in human glioblastoma through KPNA2-dependent disruption of TP53 nuclear translocation.Autophagy. 2015;11(12):2213-32. doi: 10.1080/15548627.2015.1108507. Autophagy. 2015. PMID: 26553592 Free PMC article.
-
A New Investigational Perspective for Purines Against Glioblastoma Invasiveness.Curr Drug Targets. 2018;19(16):1871-1881. doi: 10.2174/1389450119666180226123819. Curr Drug Targets. 2018. PMID: 29484991 Review.
Cited by
-
FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2.J Exp Clin Cancer Res. 2020 Sep 29;39(1):202. doi: 10.1186/s13046-020-01677-w. J Exp Clin Cancer Res. 2020. PMID: 32993749 Free PMC article.
-
Exosomal non‑coding RNAs: Novel biomarkers with emerging clinical applications in gastric cancer (Review).Mol Med Rep. 2020 Nov;22(5):4091-4100. doi: 10.3892/mmr.2020.11519. Epub 2020 Sep 17. Mol Med Rep. 2020. PMID: 33000279 Free PMC article. Review.
-
Inhibition of TGF-β1-induced epithelial-mesenchymal transition in gliomas by DMC-HA.Aging (Albany NY). 2023 Dec 27;15(24):15183-15195. doi: 10.18632/aging.205340. Epub 2023 Dec 27. Aging (Albany NY). 2023. PMID: 38154100 Free PMC article.
-
GTPBP4: A New Therapeutic Target Gene Promotes Tumor Progression in Non-Small Cell Lung Cancer via EMT.J Oncol. 2022 Nov 11;2022:2164897. doi: 10.1155/2022/2164897. eCollection 2022. J Oncol. 2022. PMID: 36405249 Free PMC article.
-
Anti-Vimentin Nanobody Decreases Glioblastoma Cell Invasion In Vitro and In Vivo.Cancers (Basel). 2023 Jan 17;15(3):573. doi: 10.3390/cancers15030573. Cancers (Basel). 2023. PMID: 36765531 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

