Cannabinoid Signaling in the Skin: Therapeutic Potential of the "C(ut)annabinoid" System
- PMID: 30845666
- PMCID: PMC6429381
- DOI: 10.3390/molecules24050918
Cannabinoid Signaling in the Skin: Therapeutic Potential of the "C(ut)annabinoid" System
Abstract
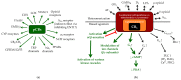
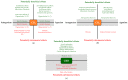
The endocannabinoid system (ECS) has lately been proven to be an important, multifaceted homeostatic regulator, which influences a wide-variety of physiological processes all over the body. Its members, the endocannabinoids (eCBs; e.g., anandamide), the eCB-responsive receptors (e.g., CB₁, CB₂), as well as the complex enzyme and transporter apparatus involved in the metabolism of the ligands were shown to be expressed in several tissues, including the skin. Although the best studied functions over the ECS are related to the central nervous system and to immune processes, experimental efforts over the last two decades have unambiguously confirmed that cutaneous cannabinoid ("c[ut]annabinoid") signaling is deeply involved in the maintenance of skin homeostasis, barrier formation and regeneration, and its dysregulation was implicated to contribute to several highly prevalent diseases and disorders, e.g., atopic dermatitis, psoriasis, scleroderma, acne, hair growth and pigmentation disorders, keratin diseases, various tumors, and itch. The current review aims to give an overview of the available skin-relevant endo- and phytocannabinoid literature with a special emphasis on the putative translational potential, and to highlight promising future research directions as well as existing challenges.
Keywords: acne; atopic dermatitis; cannabinoid; fibrosis; hair growth; inflammation; itch; psoriasis; skin; tumor; wound healing.
Conflict of interest statement
A.O. and T.B. provide consultancy services to Botanix Pharmaceuticals Ltd. (A.O.) and Phytecs Inc. (T.B.). Botanix Pharmaceuticals Ltd., Phytecs Inc., or the above founding sponsors had no role in the writing of the manuscript, or in the decision to publish it.
Figures

References
-
- Jensen J.M., Proksch E. The skin’s barrier. G. Ital. Dermatol. Venereol. 2009;144:689–700. - PubMed
-
- Oláh A., Szöllősi A.G., Bíró T. The channel physiology of the skin. Rev. Physiol. Biochem. Pharmacol. 2012;163:65–131. - PubMed
-
- Boulais N., Misery L. The epidermis: A sensory tissue. Eur. J. Dermatol. 2008;18:119–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

