Genetic Susceptibility to Human Norovirus Infection: An Update
- PMID: 30845670
- PMCID: PMC6466115
- DOI: 10.3390/v11030226
Genetic Susceptibility to Human Norovirus Infection: An Update
Abstract
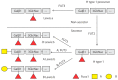
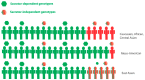
Noroviruses are the most common etiological agent of acute gastroenteritis worldwide. Despite their high infectivity, a subpopulation of individuals is resistant to infection and disease. This susceptibility is norovirus genotype-dependent and is largely mediated by the presence or absence of human histo-blood group antigens (HBGAs) on gut epithelial surfaces. The synthesis of these HBGAs is mediated by fucosyl- and glycosyltransferases under the genetic control of the FUT2 (secretor), FUT3 (Lewis) and ABO(H) genes. The so-called non-secretors, having an inactivated FUT2 enzyme, do not express blood group antigens and are resistant to several norovirus genotypes, including the predominant GII.4. Significant genotypic and phenotypic diversity of HBGA expression exists between different human populations. Here, we review previous in vivo studies on genetic susceptibility to norovirus infection. These are discussed in relation to population susceptibility, vaccines, norovirus epidemiology and the impact on public health.
Keywords: histo-blood group antigens; norovirus; secretor status; susceptibility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Becker-Dreps S., Bucardo F., Vilchez S., Zambrana L.E., Liu L., Weber D.J., Pena R., Barclay L., Vinje J., Hudgens M.G., et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: A prospective, population-based study in Nicaragua. Pediatr. Infect. Dis. J. 2014;33:1156–1163. doi: 10.1097/INF.0000000000000427. - DOI - PMC - PubMed
-
- Hemming M., Rasanen S., Huhti L., Paloniemi M., Salminen M., Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 2013;172:739–746. doi: 10.1007/s00431-013-1945-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

