Origin Firing Regulations to Control Genome Replication Timing
- PMID: 30845782
- PMCID: PMC6470937
- DOI: 10.3390/genes10030199
Origin Firing Regulations to Control Genome Replication Timing
Abstract
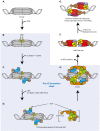
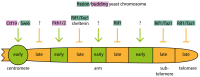
Complete genome duplication is essential for genetic homeostasis over successive cell generations. Higher eukaryotes possess a complex genome replication program that involves replicating the genome in units of individual chromatin domains with a reproducible order or timing. Two types of replication origin firing regulations ensure complete and well-timed domain-wise genome replication: (1) the timing of origin firing within a domain must be determined and (2) enough origins must fire with appropriate positioning in a short time window to avoid inter-origin gaps too large to be fully copied. Fundamental principles of eukaryotic origin firing are known. We here discuss advances in understanding the regulation of origin firing to control firing time. Work with yeasts suggests that eukaryotes utilise distinct molecular pathways to determine firing time of distinct sets of origins, depending on the specific requirements of the genomic regions to be replicated. Although the exact nature of the timing control processes varies between eukaryotes, conserved aspects exist: (1) the first step of origin firing, pre-initiation complex (pre-IC formation), is the regulated step, (2) many regulation pathways control the firing kinase Dbf4-dependent kinase, (3) Rif1 is a conserved mediator of late origin firing and (4) competition between origins for limiting firing factors contributes to firing timing. Characterization of the molecular timing control pathways will enable us to manipulate them to address the biological role of replication timing, for example, in cell differentiation and genome instability.
Keywords: eukaryotic origin firing; origin firing regulation; origin firing timing; replication timing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

