Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting
- PMID: 30845955
- PMCID: PMC6407232
- DOI: 10.1186/s13045-019-0709-6
Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting
Abstract
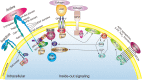
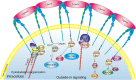
Integrins are a family of transmembrane glycoprotein signaling receptors that can transmit bioinformation bidirectionally across the plasma membrane. Integrin αIIbβ3 is expressed at a high level in platelets and their progenitors, where it plays a central role in platelet functions, hemostasis, and arterial thrombosis. Integrin αIIbβ3 also participates in cancer progression, such as tumor cell proliferation and metastasis. In resting platelets, integrin αIIbβ3 adopts an inactive conformation. Upon agonist stimulation, the transduction of inside-out signals leads integrin αIIbβ3 to switch from a low- to high-affinity state for fibrinogen and other ligands. Ligand binding causes integrin clustering and subsequently promotes outside-in signaling, which initiates and amplifies a range of cellular events to drive essential platelet functions such as spreading, aggregation, clot retraction, and thrombus consolidation. Regulation of the bidirectional signaling of integrin αIIbβ3 requires the involvement of numerous interacting proteins, which associate with the cytoplasmic tails of αIIbβ3 in particular. Integrin αIIbβ3 and its signaling pathways are considered promising targets for antithrombotic therapy. This review describes the bidirectional signal transduction of integrin αIIbβ3 in platelets, as well as the proteins responsible for its regulation and therapeutic agents that target integrin αIIbβ3 and its signaling pathways.
Keywords: Integrin αIIbβ3; Kindlin; Signal transduction; Talin; Therapeutic targeting; Transmembrane proteins.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

