Tropism and molecular pathogenesis of canine distemper virus
- PMID: 30845967
- PMCID: PMC6407191
- DOI: 10.1186/s12985-019-1136-6
Tropism and molecular pathogenesis of canine distemper virus
Abstract
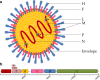
Background: Canine distemper virus (CDV), currently termed Canine morbillivirus, is an extremely contagious disease that affects dogs. It is identified as a multiple cell tropism pathogen, and its host range includes a vast array of species. As a member of Mononegavirales, CDV has a negative, single-stranded RNA genome, which encodes eight proteins.
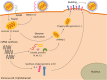
Main body: Regarding the molecular pathogenesis, the hemagglutinin protein (H) plays a crucial role both in the antigenic recognition and the viral interaction with SLAM and nectin-4, the host cells' receptors. These cellular receptors have been studied widely as CDV receptors in vitro in different cellular models. The SLAM receptor is located in lymphoid cells; therefore, the infection of these cells by CDV leads to immunosuppression, the severity of which can lead to variability in the clinical disease with the potential of secondary bacterial infection, up to and including the development of neurological signs in its later stage.
Conclusion: Improving the understanding of the CDV molecules implicated in the determination of infection, especially the H protein, can help to enhance the biochemical comprehension of the difference between a wide range of CDV variants, their tropism, and different steps in viral infection. The regions of interaction between the viral proteins and the identified host cell receptors have been elucidated to facilitate this understanding. Hence, this review describes the significant molecular and cellular characteristics of CDV that contribute to viral pathogenesis.
Keywords: Canine distemper virus; Canine morbillivirus; Molecular pathogenesis; Neuropathogenesis; Tropism; Zoonosis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Murphy FA, Fauquet CM, Bishop DH, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD. Virus taxonomy: classification and nomenclature of viruses. 2012.
-
- MacLachlan N, Dubovi E, Fenner F: Paramyxoviridae. 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

