Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord
- PMID: 30845980
- PMCID: PMC6407186
- DOI: 10.1186/s13287-019-1175-3
Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord
Abstract
Background: Mesenchymal stromal cells (MSCs) are considered potential candidates that hold great promise in the treatment of immune-related diseases. For therapeutic applications, it is necessary to isolate and expand MSCs with procedures complying with good manufacturing practice (GMP). Recent studies reported the use of human serum (HS) instead of fetal bovine serum (FBS) for the expansion of bone marrow-derived MSCs. Nevertheless, there are only limited data on HS as an alternative to FBS for the isolation and expansion of umbilical (UC-MSCs) and placenta-derived MSCs (PL-MSCs). In this study, we evaluate the effect of HS compared to FBS on the proliferative and immunosuppressive capacities of these MSCs.
Methods: PL-MSCs and UC-MSCs were isolated and cultured in HS- or FBS-supplemented media. The MSC characteristics, including morphology, immunophenotype, and differentiation ability, were verified. The proliferative and immunosuppressive capacities were also examined. In addition, the proliferative-enhancing factors in both sera were explored using proteomic analysis.
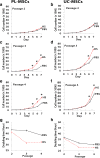
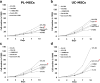
Results: PL-MSCs and UC-MSCs proliferated faster in HS-supplemented medium than in equivalent levels of FBS-supplemented medium. Adipogenic and osteogenic differentiations occurred at nearly identical levels in HS- and FBS-supplemented media. Interestingly, MSCs cultured in HS-supplemented medium had a similar immunosuppressive effect as MSCs cultured in FBS-supplemented medium. Proteomic analysis revealed that Con-A binding glycoproteins with a molecular weight > 100 kDa in FBS could significantly enhance MSC proliferation. In contrast, the proliferative enhancing factors in HS were found in the Con-A non-binding fraction and WGA binding fraction with a molecular weight > 100 kDa.
Conclusions: Taken together, our results suggest applications for the use of HS instead of FBS for the isolation and expansion of PL-MSCs and UC-MSCs for cell therapy in the future. Furthermore, this study identifies factors in HS that are responsible for its proliferative and immunosuppressive effects and might thus lead to the establishment of GMPs for the therapeutic use of MSCs.
Keywords: Con-A; Human serum; Immunosuppression; Mesenchymal stromal cell; Placenta; Umbilical cord; WGA.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Human Research Ethics Committee of Thammasat University No.I (Faculty of Medicine) and is in accordance with the declaration of Helsinki and Belmont report. All samples were obtained from donors with written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







Similar articles
-
The Biological Characteristics of Placenta Derived Mesenchymal Stem Cells Cultured in Human Serum.J Med Assoc Thai. 2016 Jul;99 Suppl 4:S75-83. J Med Assoc Thai. 2016. PMID: 29917345
-
Differential Development of Umbilical Cord-Derived Mesenchymal Stem Cells During Long-Term Maintenance in Fetal Bovine Serum-Supplemented Medium and Xeno- and Serum-Free Culture Medium.Cell Reprogram. 2021 Dec;23(6):359-369. doi: 10.1089/cell.2021.0050. Epub 2021 Nov 8. Cell Reprogram. 2021. PMID: 34748399
-
[Comparison of the biological characteristics of serum-free and fetal bovine serum-contained medium cultured umbilical cord-derived mesenchymal stem cells].Zhonghua Xue Ye Xue Za Zhi. 2012 Sep;33(9):715-9. doi: 10.3760/cma.j.issn.0253-2727.2012.09.006. Zhonghua Xue Ye Xue Za Zhi. 2012. PMID: 23336223 Chinese.
-
Human placental stem cells: biomedical potential and clinical relevance.J Stem Cells. 2011;6(2):75-92. J Stem Cells. 2011. PMID: 22997848 Review.
-
Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application.Tissue Eng Regen Med. 2021 Feb;18(1):15-23. doi: 10.1007/s13770-020-00306-z. Epub 2020 Nov 4. Tissue Eng Regen Med. 2021. PMID: 33150562 Free PMC article. Review.
Cited by
-
Biological Characteristics of Umbilical Cord Mesenchymal Stem Cells and Its Therapeutic Potential for Hematological Disorders.Front Cell Dev Biol. 2021 May 3;9:570179. doi: 10.3389/fcell.2021.570179. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34012958 Free PMC article. Review.
-
High glucose inhibits proliferation, migration, and osteogenic differentiation of human placenta-derived mesenchymal stem cells.Sci Rep. 2025 Jul 2;15(1):22512. doi: 10.1038/s41598-025-06454-3. Sci Rep. 2025. PMID: 40594898 Free PMC article.
-
Clinical Trials Targeting Secondary Damage after Traumatic Spinal Cord Injury.Int J Mol Sci. 2023 Feb 14;24(4):3824. doi: 10.3390/ijms24043824. Int J Mol Sci. 2023. PMID: 36835233 Free PMC article. Review.
-
TNF-α and IFN-γ Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles.Int J Mol Sci. 2021 Sep 2;22(17):9531. doi: 10.3390/ijms22179531. Int J Mol Sci. 2021. PMID: 34502453 Free PMC article. Review.
-
Improvement of osteogenic differentiation potential of placenta-derived mesenchymal stem cells by metformin via AMPK pathway activation.Stem Cell Res Ther. 2024 Nov 13;15(1):417. doi: 10.1186/s13287-024-04014-6. Stem Cell Res Ther. 2024. PMID: 39533406 Free PMC article.
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. - PubMed
-
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. - PubMed
-
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–398. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

