Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery
- PMID: 30846391
- PMCID: PMC6453548
- DOI: 10.1016/j.ymthe.2019.02.012
Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery
Abstract
mRNA has broad potential as a therapeutic. Current clinical efforts are focused on vaccination, protein replacement therapies, and treatment of genetic diseases. The clinical translation of mRNA therapeutics has been made possible through advances in the design of mRNA manufacturing and intracellular delivery methods. However, broad application of mRNA is still limited by the need for improved delivery systems. In this review, we discuss the challenges for clinical translation of mRNA-based therapeutics, with an emphasis on recent advances in biomaterials and delivery strategies, and we present an overview of the applications of mRNA-based delivery for protein therapy, gene editing, and vaccination.
Keywords: CRISPR; biomaterials; clinical trial; gene editing; gene therapy; lipid nanoparticles; mRNA delivery; mRNA nanoparticles; mRNA vaccine; protein replacement.
Copyright © 2019. Published by Elsevier Inc.
Figures
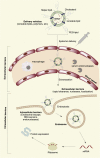
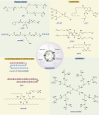

References
-
- Hajj K.A., Whitehead K.A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017;2:17056.
-
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
-
- Granot Y., Peer D. Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics-An innate immune system standpoint. Semin. Immunol. 2017;34:68–77. - PubMed
-
- Holtkamp S., Kreiter S., Selmi A., Simon P., Koslowski M., Huber C., Türeci O., Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

