Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord
- PMID: 30846445
- PMCID: PMC6602353
- DOI: 10.1242/dev.173807
Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord
Abstract
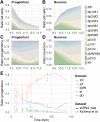
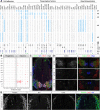
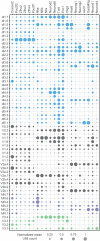
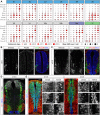
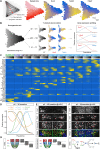
The coordinated spatial and temporal regulation of gene expression in the vertebrate neural tube determines the identity of neural progenitors and the function and physiology of the neurons they generate. Progress has been made deciphering the gene regulatory programmes that are responsible for this process; however, the complexity of the tissue has hampered the systematic analysis of the network and the underlying mechanisms. To address this, we used single cell mRNA sequencing to profile cervical and thoracic regions of the developing mouse neural tube between embryonic days 9.5-13.5. We confirmed that the data accurately recapitulates neural tube development, allowing us to identify new markers for specific progenitor and neuronal populations. In addition, the analysis highlighted a previously underappreciated temporal component to the mechanisms that generate neuronal diversity, and revealed common features in the sequence of transcriptional events that lead to the differentiation of specific neuronal subtypes. Together, the data offer insight into the mechanisms that are responsible for neuronal specification and provide a compendium of gene expression for classifying spinal cord cell types that will support future studies of neural tube development, function and disease.
Keywords: Neural development; Neural tube; ScRNA-seq; Single cell transcriptomics; Spinal cord.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

