Microenvironment tailors nTreg structure and function
- PMID: 30846549
- PMCID: PMC6442590
- DOI: 10.1073/pnas.1812471116
Microenvironment tailors nTreg structure and function
Abstract
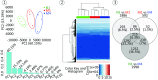
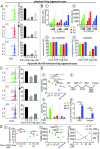
Natural regulatory T cells (nTregs) ensure the control of self-tolerance and are currently used in clinical trials to alleviate autoimmune diseases and graft-versus-host disease after hematopoietic stem cell transfer. Based on CD39/CD26 markers, blood nTreg analysis revealed the presence of five different cell subsets, each representing a distinct stage of maturation. Ex vivo added microenvironmental factors, including IL-2, TGFβ, and PGE2, direct the conversion from naive precursor to immature memory and finally from immature to mature memory cells, the latest being a no-return stage. Phenotypic and genetic characteristics of the subsets illustrate the structural parental maturation between subsets, which further correlates with the expression of regulatory factors. Regarding nTreg functional plasticity, both maturation stage and microenvironmental cytokines condition nTreg activities, which include blockade of autoreactive immune cells by cell-cell contact, Th17 and IL-10 Tr1-like activities, or activation of TCR-stimulating dendritic cell tolerization. Importantly, blood nTreg CD39/CD26 profile remained constant over a 2-y period in healthy persons but varied from person to person. Preliminary data on patients with autoimmune diseases or acute myelogenous leukemia illustrate the potential use of the nTreg CD39/CD26 profile as a blood biomarker to monitor chronic inflammatory diseases. Finally, we confirmed that naive conventional CD4 T cells, TCR-stimulated under a tolerogenic conditioned medium, could be ex vivo reprogrammed to FOXP3 lineage Tregs, and further found that these cells were exclusively committed to suppressive function under all microenvironmental contexts.
Keywords: CD39 regulatory receptor; FOXP3 regulatory transcript; adenosine deaminase-binding CD26; microenvironmental cytokines; nTregs.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: D.Z. is a cofounder of Neovacs, and R.C. is an employee of eBioscience.
Figures




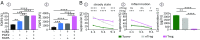
References
-
- Murphy KM, Weaver CT. Janeway’s Immunobiology. 9th Ed Garland Science; New York: 2016.
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25): Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108:586–590. - PubMed
-
- Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

