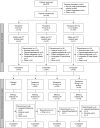
Efficacy and Safety of Tenapanor in Patients with Hyperphosphatemia Receiving Maintenance Hemodialysis: A Randomized Phase 3 Trial
- PMID: 30846557
- PMCID: PMC6442342
- DOI: 10.1681/ASN.2018080832
Efficacy and Safety of Tenapanor in Patients with Hyperphosphatemia Receiving Maintenance Hemodialysis: A Randomized Phase 3 Trial
Abstract
Background: Guidelines recommend reducing elevated serum phosphate in patients with CKD. Tenapanor, a minimally absorbed inhibitor of gastrointestinal sodium/hydrogen exchanger 3 (NHE3), reduces paracellular phosphate transport.
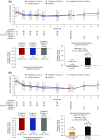
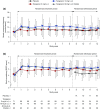
Methods: In this phase 3 randomized, double-blind trial, we randomly assigned patients with hyperphosphatemia receiving maintenance hemodialysis to receive twice-daily oral tenapanor (3, 10, or 30 mg [the latter down-titrated, if needed]) for 8 weeks. Patients were then rerandomized 1:1 to receive either their previously assigned dose or placebo for a 4-week 'withdrawal' period. We measured serum phosphate levels over the course of the trial. The primary end point was mean change in serum phosphate over the 4-week withdrawal period for the tenapanor group (using pooled data) versus the placebo group.
Results: Of 219 patients randomized, 152 completed both study phases. During the initial 8-week treatment period, all three treatment groups experienced significant decreases in mean serum phosphate (reductions of 1.00, 1.02, and 1.19 mg/dl, corresponding to the 3, 10, and 30 mg [down-titrated] dose groups, respectively). Tenapanor also showed a significant benefit over placebo during the withdrawal period, with a mean increase of 0.85 mg/dl in the placebo group versus a mean increase of 0.02 mg/dl in the pooled tenapanor group. Adverse events were largely limited to softened stool and a modest increase in bowel movement frequency, resulting from increased stool sodium and water content, stemming from tenapanor's mechanism of action.
Conclusions: Tenapanor significantly reduced elevated serum phosphate in patients with hyperphosphatemia receiving maintenance hemodialysis. Adverse effects were limited to those induced by its known mechanism of action, which increases stool sodium and water content.
Keywords: NHE3; hemodialysis; hyperphosphatemia; phosphate; tenapanor.
Copyright © 2019 by the American Society of Nephrology.
Figures


Similar articles
-
A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY).J Am Soc Nephrol. 2021 Jun 1;32(6):1465-1473. doi: 10.1681/ASN.2020101398. Epub 2021 Mar 25. J Am Soc Nephrol. 2021. PMID: 33766811 Free PMC article. Clinical Trial.
-
Effect of Tenapanor on Serum Phosphate in Patients Receiving Hemodialysis.J Am Soc Nephrol. 2017 Jun;28(6):1933-1942. doi: 10.1681/ASN.2016080855. Epub 2017 Feb 3. J Am Soc Nephrol. 2017. PMID: 28159782 Free PMC article. Clinical Trial.
-
Therapeutic Effects of Add-On Tenapanor for Hemodialysis Patients with Refractory Hyperphosphatemia.Am J Nephrol. 2021;52(6):496-506. doi: 10.1159/000516156. Epub 2021 Jun 7. Am J Nephrol. 2021. PMID: 34098559 Free PMC article. Clinical Trial.
-
Effectivity and safety profile of tenapanor, a sodium-hydrogen exchanger isoform 3 inhibitor, as an innovative treatment for hyperphosphatemia in chronic kidney disease: A systematic review of clinical studies.Nefrologia (Engl Ed). 2024 Nov-Dec;44(6):796-806. doi: 10.1016/j.nefroe.2024.11.015. Nefrologia (Engl Ed). 2024. PMID: 39701606
-
Tenapanor: A Phosphate Absorption Inhibitor for the Management of Hyperphosphatemia in Patients With Kidney Failure.J Ren Nutr. 2025 Jan;35(1):25-34. doi: 10.1053/j.jrn.2024.07.003. Epub 2024 Jul 9. J Ren Nutr. 2025. PMID: 38992521 Review.
Cited by
-
Enhanced phosphate absorption in intestinal epithelial cell-specific NHE3 knockout mice.Acta Physiol (Oxf). 2022 Feb;234(2):e13756. doi: 10.1111/apha.13756. Epub 2022 Jan 11. Acta Physiol (Oxf). 2022. PMID: 34978760 Free PMC article.
-
Oh, My Gut! New insights on the role of the gastrointestinal tract and the gut microbiome in chronic kidney disease-mineral and bone disorder.Curr Opin Nephrol Hypertens. 2024 Mar 1;33(2):226-230. doi: 10.1097/MNH.0000000000000961. Epub 2023 Dec 13. Curr Opin Nephrol Hypertens. 2024. PMID: 38088374 Free PMC article. Review.
-
NaPi-IIb Inhibition for Hyperphosphatemia in CKD Hemodialysis Patients.Kidney Int Rep. 2020 Dec 23;6(3):675-684. doi: 10.1016/j.ekir.2020.12.017. eCollection 2021 Mar. Kidney Int Rep. 2020. PMID: 33732982 Free PMC article.
-
The impact of phosphate lowering agents on clinical and laboratory outcomes in chronic kidney disease patients: a systematic review and meta-analysis of randomized controlled trials.J Nephrol. 2022 Mar;35(2):473-491. doi: 10.1007/s40620-021-01065-3. Epub 2021 Jun 1. J Nephrol. 2022. PMID: 34061337
-
Constipation in Patients With Chronic Kidney Disease.J Neurogastroenterol Motil. 2023 Oct 30;29(4):428-435. doi: 10.5056/jnm23133. J Neurogastroenterol Motil. 2023. PMID: 37814433 Free PMC article. Review.
References
-
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 - PubMed
-
- Chue CD, Edwards NC, Moody WE, Steeds RP, Townend JN, Ferro CJ: Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: A cardiac magnetic resonance study. Heart 98: 219–224, 2012 - PubMed
-
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

