The NifZ accessory protein has an equivalent function in maturation of both nitrogenase MoFe protein P-clusters
- PMID: 30846561
- PMCID: PMC6484116
- DOI: 10.1074/jbc.RA119.007905
The NifZ accessory protein has an equivalent function in maturation of both nitrogenase MoFe protein P-clusters
Abstract
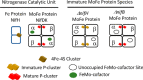
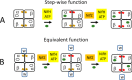
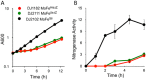
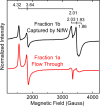
The Mo-dependent nitrogenase comprises two interacting components called the Fe protein and the MoFe protein. The MoFe protein is an α2β2 heterotetramer that harbors two types of complex metalloclusters, both of which are necessary for N2 reduction. One type is a 7Fe-9S-Mo-C-homocitrate species designated FeMo-cofactor, which provides the N2-binding catalytic site, and the other is an 8Fe-7S species designated the P-cluster, involved in mediating intercomponent electron transfer to FeMo-cofactor. The MoFe protein's catalytic partner, Fe protein, is also required for both FeMo-cofactor formation and the conversion of an immature form of P-clusters to the mature species. This latter process involves several assembly factors, NafH, NifW, and NifZ, and precedes FeMo-cofactor insertion. Here, using various protein affinity-based purification methods as well as in vivo, EPR spectroscopy, and MALDI measurements, we show that several MoFe protein species accumulate in a NifZ-deficient background of the nitrogen-fixing microbe Azotobacter vinelandii These included fully active MoFe protein replete with FeMo-cofactor and mature P-cluster, inactive MoFe protein having no FeMo-cofactor and only immature P-cluster, and partially active MoFe protein having one αβ-unit with a FeMo-cofactor and mature P-cluster and the other αβ-unit with no FeMo-cofactor and immature P-cluster. Also, NifW could associate with MoFe protein having immature P-clusters and became dissociated upon P-cluster maturation. Furthermore, both P-clusters could mature in vitro without NifZ. These findings indicate that NifZ has an equivalent, although not essential, function in the maturation of both P-clusters contained within the MoFe protein.
Keywords: Azotobacter vinelandii; FeMo-cofactor; NifW; NifZ; P-cluster; iron-sulfur protein; maturation; metalloenzyme; nitrogen fixation; nitrogen metabolism; nitrogenase.
© 2019 Jimenez-Vicente et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

