Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson's disease pathogenesis
- PMID: 30846695
- PMCID: PMC6405777
- DOI: 10.1038/s41467-019-08858-y
Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson's disease pathogenesis
Abstract
In Parkinson's disease (PD) there is a selective degeneration of neuromelanin-containing neurons, especially substantia nigra dopaminergic neurons. In humans, neuromelanin accumulates with age, the latter being the main risk factor for PD. The contribution of neuromelanin to PD pathogenesis remains unknown because, unlike humans, common laboratory animals lack neuromelanin. Synthesis of peripheral melanins is mediated by tyrosinase, an enzyme also present at low levels in the brain. Here we report that overexpression of human tyrosinase in rat substantia nigra results in age-dependent production of human-like neuromelanin within nigral dopaminergic neurons, up to levels reached in elderly humans. In these animals, intracellular neuromelanin accumulation above a specific threshold is associated to an age-dependent PD phenotype, including hypokinesia, Lewy body-like formation and nigrostriatal neurodegeneration. Enhancing lysosomal proteostasis reduces intracellular neuromelanin and prevents neurodegeneration in tyrosinase-overexpressing animals. Our results suggest that intracellular neuromelanin levels may set the threshold for the initiation of PD.
Conflict of interest statement
VHIR has applied for a use patent for the modulation of neuromelanin levels in the treatment of Parkinson's disease and/or brain aging.
Figures

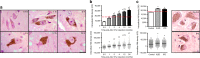


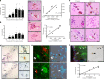
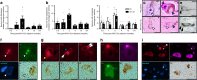
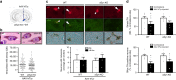

Comment in
-
Intracellular crowding by age-dependent neuromelanin accumulation disrupts neuronal proteostasis and triggers Parkinson disease pathology.Autophagy. 2019 Nov;15(11):2028-2030. doi: 10.1080/15548627.2019.1659621. Epub 2019 Sep 3. Autophagy. 2019. PMID: 31480882 Free PMC article.
Similar articles
-
FKBP51 inhibition ameliorates neurodegeneration and motor dysfunction in the neuromelanin-SNCA mouse model of Parkinson's disease.Mol Ther. 2025 Mar 5;33(3):895-916. doi: 10.1016/j.ymthe.2025.01.049. Epub 2025 Feb 3. Mol Ther. 2025. PMID: 39905728
-
Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease.Brain. 2005 Nov;128(Pt 11):2654-64. doi: 10.1093/brain/awh584. Epub 2005 Jul 6. Brain. 2005. PMID: 16000336
-
Intracellular crowding by age-dependent neuromelanin accumulation disrupts neuronal proteostasis and triggers Parkinson disease pathology.Autophagy. 2019 Nov;15(11):2028-2030. doi: 10.1080/15548627.2019.1659621. Epub 2019 Sep 3. Autophagy. 2019. PMID: 31480882 Free PMC article.
-
Toxic Feedback Loop Involving Iron, Reactive Oxygen Species, α-Synuclein and Neuromelanin in Parkinson's Disease and Intervention with Turmeric.Mol Neurobiol. 2021 Nov;58(11):5920-5936. doi: 10.1007/s12035-021-02516-5. Epub 2021 Aug 23. Mol Neurobiol. 2021. PMID: 34426907 Review.
-
Neuromelanin in Parkinson's Disease: Tyrosine Hydroxylase and Tyrosinase.Int J Mol Sci. 2022 Apr 10;23(8):4176. doi: 10.3390/ijms23084176. Int J Mol Sci. 2022. PMID: 35456994 Free PMC article. Review.
Cited by
-
Pleiotropic Potential of Evernia prunastri Extracts and Their Main Compounds Evernic Acid and Atranorin: In Vitro and In Silico Studies.Molecules. 2023 Dec 31;29(1):233. doi: 10.3390/molecules29010233. Molecules. 2023. PMID: 38202817 Free PMC article.
-
A New Rise of Non-Human Primate Models of Synucleinopathies.Biomedicines. 2021 Mar 9;9(3):272. doi: 10.3390/biomedicines9030272. Biomedicines. 2021. PMID: 33803341 Free PMC article. Review.
-
Inflammaging: The Next Challenge-Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences.Biomedicines. 2024 Aug 1;12(8):1716. doi: 10.3390/biomedicines12081716. Biomedicines. 2024. PMID: 39200181 Free PMC article. Review.
-
Advances in D-Amino Acids in Neurological Research.Int J Mol Sci. 2020 Oct 3;21(19):7325. doi: 10.3390/ijms21197325. Int J Mol Sci. 2020. PMID: 33023061 Free PMC article. Review.
-
A Novel Method for Creating a Synthetic L-DOPA Proteome and In Vitro Evidence of Incorporation.Proteomes. 2021 May 24;9(2):24. doi: 10.3390/proteomes9020024. Proteomes. 2021. PMID: 34073856 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

