Resting-state brain information flow predicts cognitive flexibility in humans
- PMID: 30846746
- PMCID: PMC6406001
- DOI: 10.1038/s41598-019-40345-8
Resting-state brain information flow predicts cognitive flexibility in humans
Abstract
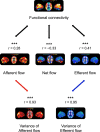
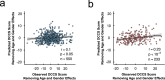
The human brain is a dynamic system, where communication between spatially distinct areas facilitates complex cognitive functions and behaviors. How information transfers between brain regions and how it gives rise to human cognition, however, are unclear. In this article, using resting-state functional magnetic resonance imaging (fMRI) data from 783 healthy adults in the Human Connectome Project (HCP) dataset, we map the brain's directed information flow architecture through a Granger-Geweke causality prism. We demonstrate that the information flow profiles in the general population primarily involve local exchanges within specialized functional systems, long-distance exchanges from the dorsal brain to the ventral brain, and top-down exchanges from the higher-order systems to the primary systems. Using an information flow map discovered from 550 subjects, the individual directed information flow profiles can significantly predict cognitive flexibility scores in 233 novel individuals. Our results provide evidence for directed information network architecture in the cerebral cortex, and suggest that features of the information flow configuration during rest underpin cognitive ability in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Rosenberg, M. D. et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci19, 165–171, 10.1038/nn.4179, http://www.nature.com/neuro/journal/v19/n1/abs/nn.4179.html - supplementary-information (2016). - PMC - PubMed
-
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Human brain mapping. 1994;2:56–78. doi: 10.1002/hbm.460020107. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

