The evolving role of T-bet in resistance to infection
- PMID: 30846856
- PMCID: PMC7272213
- DOI: 10.1038/s41577-019-0145-4
The evolving role of T-bet in resistance to infection
Abstract
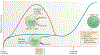
The identification of T-bet as a key transcription factor associated with the development of IFNγ-producing CD4+ T cells predicted a crucial role for T-bet in cell-mediated immunity and in resistance to many intracellular infections. This idea was reinforced by initial reports showing that T-bet-deficient mice were more susceptible to pathogens that survived within the lysosomal system of macrophages. However, subsequent studies revealed IFNγ-dependent, T-bet-independent pathways of resistance to diverse classes of microorganisms that occupy other intracellular niches. Consequently, a more complex picture has emerged of how T-bet and the related transcription factor eomesodermin (EOMES) coordinate many facets of the immune response to bona fide pathogens as well as commensals. This article provides an overview of the discovery and evolutionary relationship between T-bet and EOMES and highlights the studies that have uncovered broader functions of T-bet in innate and adaptive immunity and in the development of the effector and memory T cell populations that mediate long-term resistance to infection.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
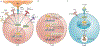
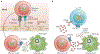

References
-
- Miller JF & Mitchell GF The thymus and the precursors of antigen reactive cells. Nature 216, 659–663 (1967). - PubMed
-
- Miller JF Discovering the origins of immunological competence. Annu. Rev. Immunol 17, 1–17 (1999). - PubMed
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA & Coffman RL Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol 136, 2348–2357 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

