Hydrolyzation of mogrosides: Immobilized β-glucosidase for mogrosides deglycosylation from Lo Han Kuo
- PMID: 30847162
- PMCID: PMC6392867
- DOI: 10.1002/fsn3.932
Hydrolyzation of mogrosides: Immobilized β-glucosidase for mogrosides deglycosylation from Lo Han Kuo
Abstract
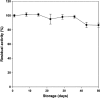
An immobilized enzyme system for bioconversion of Lo Han Kuo (LHK) mogrosides was established. β-Glucosidase which was covalently immobilized onto the glass spheres exhibited a significant bioconversion efficiency from pNPG to pnitrophenol over other carriers. Optimum operational pH and temperature were determined to be pH 4 and 30°C. Results of storage stability test demonstrated that the glass sphere enzyme immobilization system was capable of sustaining more than 80% residual activity until 50 days, and operation reusability was confirmed for at least 10 cycles. The Michaelis constant (K m) of the system was determined to be 0.33 mM. The kinetic parameters, rate constant (K) at which Mogrosides conversion was determined, the τ 50 in which 50% of mogroside V deglycosylation/mogroside IIIE production was reached, and the τ complete of complete mogroside V deglycosylation/mogroside IIIE production, were 0.044/0.017 min-1, 15.6/41.1 min, and 60/120 min, respectively. Formation of the intermediates contributed to the kinetic differences between mogroside V deglycosylation and mogroside IIIE formation.
Keywords: Lo Han Kuo; glucosidase; immobilized enzyme; mogroside IIIE; mogroside V.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures







Similar articles
-
Enrichment of two isoflavone aglycones in black soymilk by immobilized β-glucosidase on solid carriers.J Agric Food Chem. 2012 Dec 26;60(51):12540-6. doi: 10.1021/jf304405t. Epub 2012 Dec 13. J Agric Food Chem. 2012. PMID: 23190054
-
Insulin secretion stimulating effects of mogroside V and fruit extract of luo han kuo (Siraitia grosvenori Swingle) fruit extract.Yao Xue Xue Bao. 2009 Nov;44(11):1252-7. Yao Xue Xue Bao. 2009. PMID: 21351724
-
Enzymatic hydrolyzation of mogrosides in Luo Han Guo extract by NKA-adsorbed snailase improves its sensory profile.Food Chem. 2022 Oct 1;390:133205. doi: 10.1016/j.foodchem.2022.133205. Epub 2022 May 13. Food Chem. 2022. PMID: 35598415
-
Bibliometric analysis on the literature of monk fruit extract and mogrosides as sweeteners.Front Nutr. 2023 Aug 29;10:1253255. doi: 10.3389/fnut.2023.1253255. eCollection 2023. Front Nutr. 2023. PMID: 37706210 Free PMC article.
-
[Synthetic biology for the synthesis of mogroside V - a review].Sheng Wu Gong Cheng Xue Bao. 2020 Oct 25;36(10):2017-2028. doi: 10.13345/j.cjb.200072. Sheng Wu Gong Cheng Xue Bao. 2020. PMID: 33169567 Review. Chinese.
Cited by
-
Production of Siamenoside I and Mogroside IV from Siraitia grosvenorii Using Immobilized β-Glucosidase.Molecules. 2022 Sep 26;27(19):6352. doi: 10.3390/molecules27196352. Molecules. 2022. PMID: 36234889 Free PMC article.
-
Post-Ripening and Key Glycosyltransferase Catalysis to Promote Sweet Mogrosides Accumulation of Siraitia grosvenorii Fruits.Molecules. 2023 Jun 11;28(12):4697. doi: 10.3390/molecules28124697. Molecules. 2023. PMID: 37375251 Free PMC article.
-
Construction and Optimization of the de novo Biosynthesis Pathway of Mogrol in Saccharomyces Cerevisiae.Front Bioeng Biotechnol. 2022 May 27;10:919526. doi: 10.3389/fbioe.2022.919526. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35711645 Free PMC article.
-
Status of the application of exogenous enzyme technology for the development of natural plant resources.Bioprocess Biosyst Eng. 2021 Mar;44(3):429-442. doi: 10.1007/s00449-020-02463-w. Epub 2020 Nov 4. Bioprocess Biosyst Eng. 2021. PMID: 33146790 Review.
-
Assessing Edible Filamentous Fungal Carriers as Cell Supports for Growth of Yeast and Cultivated Meat.Foods. 2022 Oct 9;11(19):3142. doi: 10.3390/foods11193142. Foods. 2022. PMID: 36230217 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

