Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: Role of autophagy
- PMID: 30847250
- PMCID: PMC6389652
- DOI: 10.1016/j.jare.2019.01.013
Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: Role of autophagy
Abstract
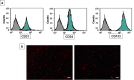
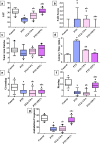
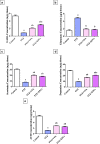
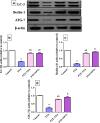
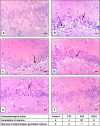
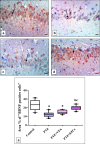
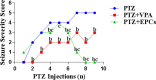
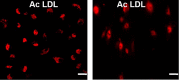
Epilepsy is one of the most well-known neurological conditions worldwide. One-third of adult epileptic patients do not respond to antiepileptic drugs or surgical treatment and therefore suffer from the resistant type of epilepsy. Stem cells have been given substantial consideration in the field of epilepsy therapeutics. The implication of pathologic vascular response in sustained seizures and the eminent role of endothelial progenitor cells (EPCs) in maintaining vascular integrity tempted us to investigate the potential therapeutic effects of EPCs in a pentylenetetrazole (PTZ)-induced rat model of epilepsy. Modulation of autophagy, a process that enables neurons to maintain an equilibrium of synthesis, degradation and subsequent reprocessing of cellular components, has been targeted. Intravenously administered EPCs homed into the hippocampus and amended the deficits in memory and locomotor activity. The cells mitigated neurological damage and the associated histopathological alterations and boosted the expression of brain-derived neurotrophic factor. EPCs corrected the perturbations in neurotransmitter activity and enhanced the expression of the downregulated autophagy proteins light chain protein-3 (LC-3), beclin-1, and autophagy-related gene-7 (ATG-7). Generally, these effects were comparable to those achieved by the reference antiepileptic drug, valproic acid. In conclusion, EPCs may confer therapeutic effects against epilepsy and its associated behavioural and biochemical abnormalities at least in part via the upregulation of autophagy. The study warrants further research in experimental and clinical settings to verify the prospect of using EPCs as a valid therapeutic strategy in patients with epilepsy.
Keywords: Autophagy; Endothelial progenitor cells; Epilepsy; Neuronal damage; Pentylenetetrazole.
Figures

References
-
- WHO. WHO | Epilepsy Fact sheet fs999; 2017.
-
- Verma A., Kumar A. Sudden unexpected death in epilepsy: some approaches for its prevention and medico-legal consideration. Acta Neurol Belg. 2015;115(3):207–212. - PubMed
-
- Quintas R., Raggi A., Giovannetti A.M., Pagani M., Sabariego C., Cieza A. Psychosocial difficulties in people with epilepsy: a systematic review of literature from 2005 until 2010. Epilepsy Behav. 2012;25(1):60–67. - PubMed
-
- Gan J., Qu Y., Li J., Zhao F., Mu D. An evaluation of the links between microRNA, autophagy, and epilepsy. Rev Neurosci. 2015;26(2):225–237. - PubMed
LinkOut - more resources
Full Text Sources

