Neutrophil Subsets, Platelets, and Vascular Disease in Psoriasis
- PMID: 30847414
- PMCID: PMC6390681
- DOI: 10.1016/j.jacbts.2018.10.008
Neutrophil Subsets, Platelets, and Vascular Disease in Psoriasis
Abstract
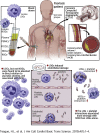
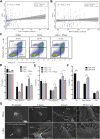
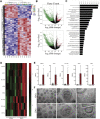
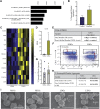
Psoriasis is an inflammatory skin disease associated with increased cardiovascular risk and serves as a reliable model to study inflammatory atherogenesis. Because neutrophils are implicated in atherosclerosis development, this study reports that the interaction among low-density granulocytes, a subset of neutrophils, and platelets is associated with a noncalcified coronary plaque burden assessed by coronary computed tomography angiography. Because early atherosclerotic noncalcified burden can lead to fatal myocardial infarction, the low-density granulocyte-platelet interaction may play a crucial target for clinical intervention.
Keywords: CCTA, coronary computed tomography angiography; CVD, cardiovascular disease; FDR, false discovery rate; HAoEC, human aortic endothelial cell; LDG, low-density granulocyte; MI, myocardial infarction; NCB, noncalcified coronary plaque burden; NDG, normal-density granulocyte; NET, neutrophil extracellular trap; PASI, psoriasis area severity index; SLE, systemic lupus erythematosus; TB, total coronary plaque burden; cardiovascular disease; low-density granulocytes; neutrophils; platelets; psoriasis.
Figures


References
-
- Prodanovich S., Kirsner R.S., Kravetz J.D., Ma F., Martinez L., Federman D.G. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. - PubMed
-
- Rachakonda T.D., Schupp C.W., Armstrong A.W. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. - PubMed
-
- Meyer N., Paul C., Feneron D. Psoriasis: an epidemiological evaluation of disease burden in 590 patients. J Eur Acad Dermatol Venereol. 2010;24:1075–1082. - PubMed
-
- Brauchli Y.B., Jick S.S., Miret M., Meier C.R. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol. 2009;160:1048–1056. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

