Mitochondria Released by Apoptotic Cell Death Initiate Innate Immune Responses
- PMID: 30847435
- PMCID: PMC6400482
- DOI: 10.4049/immunohorizons.1800063
Mitochondria Released by Apoptotic Cell Death Initiate Innate Immune Responses
Erratum in
-
Mitochondria Released by Apoptotic Cell Death Initiate Innate Immune Responses.Immunohorizons. 2019 Jan;3(1):26-27. doi: 10.4049/immunohorizons.1800089. Immunohorizons. 2019. PMID: 30911736 Free PMC article. No abstract available.
Abstract
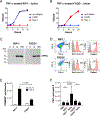
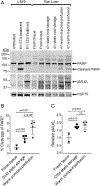
In solid organ transplantation, cell death arising from ischemia/reperfusion leads to the release of several damage-associated molecular patterns derived from mitochondria. Mitochondrial damage-associated molecular patterns (mtDAMPs) initiate proinflammatory responses, but it remains unknown whether the mode of cell death affects the inflammatory properties of mitochondria. Murine and human cell lines induced to selectively undergo apoptosis and necroptosis were used to examine the extracellular release of mitochondria during programmed cell death. Mitochondria purified from healthy, apoptotic, and necroptotic cells were used to stimulate macrophage inflammasome responses in vitro and neutrophil chemotaxis in vivo. Inhibition of specific mtDAMPs was performed to identify those responsible for macrophage inflammasome activation. A rat liver transplant model was used to identify apoptotic and necroptotic cell death in graft tissue following ischemia/reperfusion. Both apoptotic and necroptotic cell death occur in parallel in graft tissue. Apoptotic cells released more mitochondria than necroptotic cells. Moreover, mitochondria from apoptotic cells were significantly more inflammatory in terms of macrophage inflammasome activation and neutrophil recruitment. Inhibition of cellular synthesis of cardiolipin, a mitochondria-specific lipid and mtDAMP, significantly reduced the inflammasome-activating properties of apoptosis-derived mitochondria. Mitochondria derived from apoptotic cells are potent activators of innate immune responses, whereas mitochondria derived from healthy or necroptotic cells are significantly less inflammatory. Cardiolipin appears to be a key mtDAMP-regulating inflammasome activation by mitochondria. Methods of inhibiting apoptotic cell death in transplant grafts may be beneficial for reducing graft inflammation and transplant allosensitization.
Conflict of interest statement
DISCLOSURES The authors have no financial conflicts of interest.
Figures





References
-
- Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, and Faist E 2001. Influence of brain death on cytokine release in organ donors and renal transplants. Transplant. Proc 33: 1284–1285. - PubMed
-
- Murugan R, Venkataraman R, Wahed AS, Elder M, Hergenroeder G, Carter M, Madden NJ, Powner D, and Kellum JA, HIDonOR Study Investigators. 2008. Increased plasma interleukin-6 in donors is associated with lower recipient hospital-free survival after cadaveric organ transplantation. Crit. Care Med 36: 1810–1816. - PubMed
-
- Barklin A 2009. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol. Scand 53: 425–435. - PubMed
-
- Ioannou A, Dalle Lucca J, and Tsokos GC 2011. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin. Immunol 141: 3–14. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

