Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib
- PMID: 30847464
- PMCID: PMC7360149
- DOI: 10.1093/annonc/mdz077
Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib
Abstract
Background: Concurrent programmed death-ligand-1 (PD-(L)1) plus osimertinib is associated with severe immune related adverse events (irAE) in epidermal growth factor receptor (EGFR)-mutant non-small-cell lung cancer (NSCLC). Now that PD-(L)1 inhibitors are routinely used as adjuvant and first-line treatments, sequential PD-(L)1 inhibition followed by osimertinib use may become more frequent and have unforeseen serious toxicity.
Methods: We identified patients with EGFR-mutant NSCLC who were treated with PD-(L)1 blockade and EGFR- tyrosine kinase inhibitors (TKIs), irrespective of drug or sequence of administration (total n = 126). Patient records were reviewed to identify severe (NCI-CTCAE v5.0 grades 3-4) toxicity.
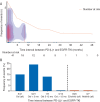
Results: Fifteen percent [6 of 41, 95% confidence interval (CI) 7% to 29%] of all patients treated with sequential PD-(L)1 blockade followed later by osimertinib developed a severe irAE. Severe irAEs were most common among those who began osimertinib within 3 months of prior PD-(L)1 blockade (5 of 21, 24%, 95% CI 10% to 45%), as compared with >3-12 months (1 of 8, 13%, 95% CI 0% to 50%), >12 months (0 of 12, 0%, 95% CI 0% to 28%). By contrast, no severe irAEs were identified among patients treated with osimertinib followed by PD-(L)1 (0 of 29, 95% CI 0% to 14%) or PD-(L)1 followed by other EGFR-TKIs (afatinib or erlotinib, 0 of 27, 95% CI 0% to 15%). IrAEs occurred at a median onset of 20 days after osimertinib (range 14-167 days). All patients with irAEs required steroids and most required hospitalization.
Conclusion: PD-(L)1 blockade followed by osimertinib is associated with severe irAE and is most frequent among patients who recently received PD-(L)1 blockade. No irAEs were observed when osimertinib preceded PD-(L)1 blockade or when PD-(L)1 was followed by other EGFR-TKIs. This association appears to be specific to osimertinib, as no severe irAEs occurred with administration of other EGFR-TKIs.
Keywords: EGFR; PD-1; TKI; irAE; osimertinib.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. - PubMed
-
- Gandhi L., Rodríguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:1689–1699. - PubMed
-
- Reck M., Rodriguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. - PubMed
-
- Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. - PubMed
-
- Ribas A., Hodi F.S., Callahan M. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–1366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

