Serum and nutrient deprivation increase autophagic flux in intervertebral disc annulus fibrosus cells: an in vitro experimental study
- PMID: 30847707
- PMCID: PMC6538458
- DOI: 10.1007/s00586-019-05910-9
Serum and nutrient deprivation increase autophagic flux in intervertebral disc annulus fibrosus cells: an in vitro experimental study
Abstract
Purpose: The loss of nutrient supply is a suspected contributor of intervertebral disc degeneration. However, the extent to which low nutrition affects disc annulus fibrosus (AF) cells is unknown as nutrient deprivation has mainly been investigated in disc nucleus pulposus cells. Hence, an experimental study was designed to clarify the effects of limited nutrients on disc AF cell fate, including autophagy, the process by which cells recycle their own damaged components.
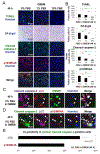
Methods: Rabbit disc AF cells were cultured in different media with varying serum concentrations under 5% oxygen. Cellular responses to changes in serum and nutrient concentrations were determined by measuring proliferation and metabolic activity. Autophagic flux in AF cells was longitudinally monitored using imaging cytometry and Western blotting for LC3, HMGB1, and p62/SQSTM1. Apoptosis (TUNEL staining and cleaved caspase-3 immunodetection) and cellular senescence (senescence-associated β-galactosidase assay and p16/INK4A immunodetection) were measured.
Results: Markers of apoptosis and senescence increased, while cell proliferation and metabolic activity decreased under the withdrawal of serum and of nutrients other than oxygen, confirming cellular stress. Time-dependent increases in autophagy markers, including LC3 puncta number per cell, LC3-II expression, and cytoplasmic HMGB1, were observed under conditions of reduced nutrition, while an autophagy substrate, p62/SQSTM1, decreased over time. Collectively, these findings suggest increased autophagic flux in disc AF cells under serum and nutrient deprivation.
Conclusion: Disc AF cells exhibit distinct responses to serum and nutrient deprivation. Cellular responses include cell death and quiescence in addition to reduced proliferation and metabolic activity, as well as activation of autophagy under conditions of nutritional stress. These slides can be retrieved under Electronic Supplementary Material.
Keywords: Annulus fibrosus (AF) cells; Apoptosis; Autophagy; Intervertebral disc; Senescence; Serum and nutrient deprivation.
Conflict of interest statement
CONFLICT OF INTEREST
TY, WB, HM, RH, KT, YK, KN, MK, NV, ML, and GS have no conflicts of interest to declare. JDK has received research grants from Stryker and Synthes.
Figures




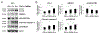
References
-
- Urban JP, Smith S, Fairbank JC (2004) Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 29 (23):2700–2709 - PubMed
-
- Yurube T, Hirata H, Kakutani K, Maeno K, Takada T, Zhang Z, Takayama K, Matsushita T, Kuroda R, Kurosaka M, Nishida K (2014) Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther 16 (1):R31. doi:10.1186/ar4460 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

