ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs
- PMID: 30847984
- PMCID: PMC6524099
- DOI: 10.1111/jvim.15463
ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs
Abstract
Immune-mediated hemolytic anemia (IMHA) causes severe anemia in dogs and is associated with considerable morbidity and mortality. Treatment with various immunosuppressive and antithrombotic drugs has been described anecdotally and in previous studies, but little consensus exists among veterinarians as to the optimal regimen to employ and maintain after diagnosis of the disease. To address this inconsistency and provide evidence-based guidelines for treatment of IMHA in dogs, we identified and extracted data from studies published in the veterinary literature. We developed a novel tool for evaluation of evidence quality, using it to assess study design, diagnostic criteria, explanation of treatment regimens, and validity of statistical methods. In combination with our clinical experience and comparable guidelines for humans afflicted with autoimmune hemolytic anemia, we used the conclusions of this process to make a set of clinical recommendations regarding treatment of IMHA in dogs, which we refined subsequently by conducting several iterations of Delphi review. Additionally, we considered emerging treatments for IMHA in dogs and highlighted areas deserving of future research. Comments were solicited from several professional bodies to maximize clinical applicability before the recommendations were submitted for publication. The resulting document is intended to provide clinical guidelines for management of IMHA in dogs. These guidelines should be implemented pragmatically, with consideration of animal, owner, and veterinary factors that may vary among cases.
Keywords: AIHA; IMHA; azathioprine; canine; cyclosporine; guideline; mycophenolate mofetil; prednisolone.
© 2019 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
Andrew Mackin is associated with the Mississippi State University Pharmacodynamic Laboratory, which offers therapeutic drug monitoring of cyclosporine as a commercial assay. All other authors had no conflicts of interest to declare.
Figures
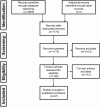



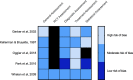



References
-
- Lewis RM, Schwartz RS, Gilmore CE. Autoimmune diseases in domestic animals. Ann N Y Acad Sci. 1965;124:178‐200. - PubMed
-
- Swann JW, Skelly BJ. Systematic review of evidence relating to the treatment of immune‐mediated hemolytic anemia in dogs. J Vet Intern Med. 2013;27:1‐9. - PubMed
-
- Miller SA, Hohenhaus AE, Hale AS. Case‐control study of blood type, breed, sex, and bacteremia in dogs with immune‐mediated hemolytic anemia. J Am Vet Med Assoc. 2004;224:232‐235. - PubMed
-
- Swann JW, Skelly BJ. Evaluation of immunosuppressive regimens for immune‐mediated haemolytic anaemia: a retrospective study of 42 dogs. J Small Anim Pract. 2011;52:353‐358. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

