Tisagenlecleucel Model-Based Cellular Kinetic Analysis of Chimeric Antigen Receptor-T Cells
- PMID: 30848084
- PMCID: PMC6539725
- DOI: 10.1002/psp4.12388
Tisagenlecleucel Model-Based Cellular Kinetic Analysis of Chimeric Antigen Receptor-T Cells
Abstract
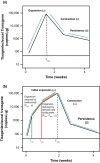
Tisagenlecleucel is a chimeric antigen receptor-T cell therapy that facilitates the killing of CD19+ B cells. A model was developed for the kinetics of tisagenlecleucel and the impact of therapies for treating cytokine release syndrome (tocilizumab and corticosteroids) on expansion. Data from two phase II studies in pediatric and young adult relapsed/refractory B cell acute lymphoblastic leukemia were pooled to evaluate this model and evaluate extrinsic and intrinsic factors that may impact the extent of tisagenlecleucel expansion. The doubling time, initial decline half-life, and terminal half-life for tisagenlecleucel were 0.78, 4.3, and 220 days, respectively. No impact of tocilizumab or corticosteroids on the expansion rate was observed. This work represents the first mixed-effect model-based analysis of chimeric antigen receptor-T cell therapy and may be clinically impactful as future studies examine prophylactic interventions in patients at risk of higher grade cytokine release syndrome and the effects of these interventions on chimeric antigen receptor-T cell expansion.
© 2019 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.M.S. is an employee of Novartis Institutes for BioMedical Research and owns equity in Novartis Pharmaceuticals Corporation. S.A.G. received consultancy fees from Novartis Pharmaceuticals Corporation, Jazz Pharmaceuticals, Adaptimmune and research support from Novartis Pharmaceuticals Corporation. J.E.L. received consultancy fees from Novartis Pharmaceuticals Corporation, Jazz Pharmaceuticals, Bluebird Bio, and Therakos, Inc. and holds patents, royalties, or intellectual property with Viracor. T.W.L. received consultancy fees from Novartis Pharmaceuticals Corporation, Loxo Oncology, and Eli Lilly and received researching funding from Pfizer. M.A.P. received consultancy fees from Novartis Pharmaceuticals Corporation and Jazz Pharmaceuticals and received travel accommodations or expenses from Medac and research funding from Adaptive Biotechnologies. M.W.B. received honoraria from Novartis Pharmaceuticals Corporation. K.J.A. participated in a speaker bureau for and received travel accommodations and expenses from Novartis Pharmaceuticals Corporation. B.L.L. received consultancy fees from GE Health and Brammer Bio, has patents and royalties with and received research funding from Novartis Pharmaceuticals Corporation, and holds equity ownership in and received research funding from Tmunity Therapeutics. L.T., S.S., M.L., and P.H.H. are employees of Novartis Pharmaceuticals Corporation and own equity in Novartis Pharmaceuticals Corporation. R.A. is an employee of Novartis Institutes for BioMedical Research and holds stock or equity ownership in Exelixis, Cara Therapeutics, Ultragenyx, and Aeterna Zentari. K.T.M. is an employee of Novartis Institutes for BioMedical Research and owns equity in Novartis Pharmaceuticals Corporation and has patents pending related to the submitted work. P.A.W. is a former employee of Novartis Pharmaceuticals Corporation and owns equity in Novartis Pharmaceuticals Corporation. C.H.J. received research support from Novartis Pharmaceuticals Corporation, received honoraria from and is a member on the board of directors or advisory committee for Western Institutional Review Board (WIRB) Copernicus Group and Celldex, owns equity in and is a member on a board of directors or advisory committee for Immune Design, has patents and royalties with Novartis Pharmaceuticals Corporation, and received research funding from Tmunity Therapeutics.
Figures



References
-
- Buechner, J. et al Global registration trial of efficacy and safety of CTL019 in pediatric and young adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL): update to the interim analysis. Clin. Lymphoma Myeloma Leuk. 17, S263–S264 (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

