Clinical validation of an immunohistochemistry-based CanAssist-Breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients
- PMID: 30848103
- PMCID: PMC6488210
- DOI: 10.1002/cam4.2049
Clinical validation of an immunohistochemistry-based CanAssist-Breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients
Abstract
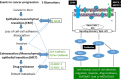
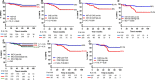
CanAssist-Breast (CAB) is an immunohistochemistry (IHC)-based prognostic test for early-stage Hormone Receptor (HR+)-positive breast cancer patients. CAB uses a Support Vector Machine (SVM) trained algorithm which utilizes expression levels of five biomarkers (CD44, ABCC4, ABCC11, N-Cadherin, and Pan-Cadherin) and three clinical parameters such as tumor size, grade, and node status as inputs to generate a risk score and categorizes patients as low- or high-risk for distant recurrence within 5 years of diagnosis. In this study, we present clinical validation of CAB. CAB was validated using a retrospective cohort of 857 patients. All patients were treated either with endocrine therapy or chemoendocrine therapy. Risk categorization by CAB was analyzed by calculating Distant Metastasis-Free Survival (DMFS) and recurrence rates using Kaplan-Meier survival curves. Multivariate analysis was performed to calculate Hazard ratios (HR) for CAB high-risk vs low-risk patients. The results showed that Distant Metastasis-Free Survival (DMFS) was significantly different (P-0.002) between low- (DMFS: 95%) and high-risk (DMFS: 80%) categories in the endocrine therapy treated alone subgroup (n = 195) as well as in the total cohort (n = 857, low-risk DMFS: 95%, high-risk DMFS: 84%, P < 0.0001). In addition, the segregation of the risk categories was significant (P = 0.0005) in node-positive patients, with a difference in DMFS of 12%. In multivariate analysis, CAB risk score was the most significant predictor of distant recurrence with hazard ratio of 3.2048 (P < 0.0001). CAB stratified patients into discrete risk categories with high statistical significance compared to Ki-67 and IHC4 score-based stratification. CAB stratified a higher percentage of the cohort (82%) as low-risk than IHC4 score (41.6%) and could re-stratify >74% of high Ki-67 and IHC4 score intermediate-risk zone patients into low-risk category. Overall the data suggest that CAB can effectively predict risk of distant recurrence with clear dichotomous high- or low-risk categorization.
Keywords: CanAssist-Breast; distant recurrence; early-stage breast cancer; immunohistochemistry; prognostication; support vector machine.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors are employees/consultants at OncoStem Diagnostics Private Limited which has developed the CanAssist‐Breast test. MMB and CR are co‐inventors on a patent application related to this article. All other authors have no other competing interests to declare.
Figures


References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomized trials. Lancet. 2005;365:1687‐1717. - PubMed
-
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen‐treated, node‐negative breast cancer. N Engl J Med. 2004;351:2817‐2826. - PubMed
-
- van de Vijver M, He Y, Veer LJ, et al. A gene‐expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999‐2009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

