Mycobacterium tuberculosis: Bacterial Fitness within the Host Macrophage
- PMID: 30848232
- PMCID: PMC6459685
- DOI: 10.1128/microbiolspec.BAI-0001-2019
Mycobacterium tuberculosis: Bacterial Fitness within the Host Macrophage
Abstract
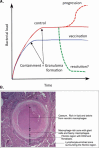
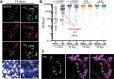
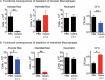
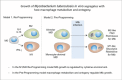
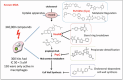
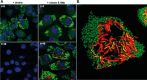
Mycobacterium tuberculosis has evolved to become the single greatest cause of death from an infectious agent. The pathogen spends most of its infection cycle in its human host within a phagocyte. The bacterium has evolved to block the normal maturation and acidification of its phagosome and resides in a vacuole contiguous with the early endosomal network. Cytokine-mediated activation of the host cell can overcome this blockage, and an array of antimicrobial responses can limit its survival. The survival of M. tuberculosis in its host cell is fueled predominantly by fatty acids and cholesterol. The ability of M. tuberculosis to degrade sterols is an unusual metabolic characteristic that was likely retained from a saprophytic ancestor. Recent results with fluorescent M. tuberculosis reporter strains demonstrate that bacterial survival differs with the host macrophage population. Tissue-resident alveolar macrophages, which are biased towards an alternatively activated, M2-like phenotype, are more permissive to bacterial growth than monocyte-derived, inflammatory, M1-like interstitial macrophages. The differential growth of the bacterium in these different phagocyte populations appears to be linked to host cell metabolism.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

