The Intracellular Life Cycle of Brucella spp
- PMID: 30848234
- PMCID: PMC6448592
- DOI: 10.1128/microbiolspec.BAI-0006-2019
The Intracellular Life Cycle of Brucella spp
Abstract
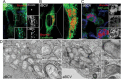
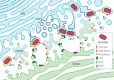
Bacteria of the genus Brucella colonize a wide variety of mammalian hosts, in which their infectious cycle and ability to cause disease predominantly rely on an intracellular lifestyle within phagocytes. Upon entry into host cells, Brucella organisms undergo a complex, multistage intracellular cycle in which they sequentially traffic through, and exploit functions of, the endocytic, secretory, and autophagic compartments via type IV secretion system (T4SS)-mediated delivery of bacterial effectors. These effectors modulate an array of host functions and machineries to first promote conversion of the initial endosome-like Brucella-containing vacuole (eBCV) into a replication-permissive organelle derived from the host endoplasmic reticulum (rBCV) and then to an autophagy-related vacuole (aBCV) that mediates bacterial egress. Here we detail and discuss our current knowledge of cellular and molecular events of the Brucella intracellular cycle. We discuss the importance of the endosomal stage in determining T4SS competency, the roles of autophagy in rBCV biogenesis and aBCV formation, and T4SS-driven mechanisms of modulation of host secretory traffic in rBCV biogenesis and bacterial egress.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

