Methodology for the establishment of primary porcine vocal fold epithelial cell cultures
- PMID: 30848488
- PMCID: PMC6779414
- DOI: 10.1002/lary.27909
Methodology for the establishment of primary porcine vocal fold epithelial cell cultures
Abstract
Objective: A current lack of methods for epithelial cell culture significantly hinders our understanding of the role of the epithelial and mucus barriers in vocal fold health and disease. Our first objective was to establish reproducible techniques for the isolation and culture of primary porcine vocal fold epithelial cells. Our second objective was to evaluate the functional significance of cell cultures using an in vitro exposure to an inflammatory cytokine.
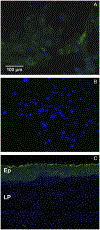
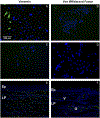
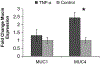
Methods: Epithelial cells were isolated from porcine vocal folds and expanded in culture. Characterization of cultures was completed by immunostaining with markers for pan-cytokeratin (epithelial cells), vimentin (stromal cells), von Willebrand factor (endothelial cell), and MUC1 and MUC4 (mucin) glycoproteins. Established epithelial cell cultures were then exposed to the inflammatory cytokine tumor necrosis factor alpha (TNF-α) for 24-hours, and transcript expression of MUC1 and MUC4 was evaluated.
Results: Reproducible, porcine vocal fold epithelial cell cultures, demonstrating cobblestone appearance characteristic of the typical morphology of epithelial cell cultures were created. Cells showed positive staining for pan-cytokeratin with limited expression of vimentin and von Willebrand factor. Epithelial cells also expressed MUC1 and MUC4. TNF-α significantly increased transcript expression of MUC4.
Conclusion: Here, we present the first report of successful culture of primary porcine vocal fold epithelial cells. Cultures will provide researchers with a valuable new in vitro tool to investigate vocal fold epithelium and mucus as well as the effects of common challenges, including inflammatory cytokines, on these barriers.
Level of evidence: NA Laryngoscope, 129:E355-E364, 2019.
Keywords: Vocal fold; cell culture; epithelium; mucin; porcine.
© 2019 The American Laryngological, Rhinological and Otological Society, Inc.
Figures




References
-
- Samuels T, Handler E, Syring N, Blumin J, Kerschner J, Johnston N. Mucin gene expression in human laryngeal epithelia: effect of laryngopharyngeal reflux. Ann Otol Rhinol Laryngol 2008;117:688–695. - PubMed
-
- Gill G, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: Investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol 2005;114:913–921. - PubMed
-
- Dworkin JP. Laryngitis: types, causes, and treatments. Otolaryngol Clin North Am 2008;41:419–436, ix. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

