A Fifty-Two-Week, Randomized, Placebo-Controlled Trial of Certolizumab Pegol in Nonradiographic Axial Spondyloarthritis
- PMID: 30848558
- PMCID: PMC6619287
- DOI: 10.1002/art.40866
A Fifty-Two-Week, Randomized, Placebo-Controlled Trial of Certolizumab Pegol in Nonradiographic Axial Spondyloarthritis
Abstract
Objective: The natural history of nonradiographic axial spondyloarthritis (SpA) is incompletely characterized, and there are concerns that nonsteroidal antiinflammatory drugs provide inadequate disease control in patients with active disease. This study was undertaken to investigate the effects of certolizumab pegol (CZP), an anti-tumor necrosis factor treatment, in patients with nonradiographic axial SpA with objective signs of inflammation.
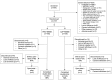
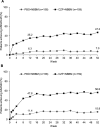
Methods: In this ongoing parallel-group double-blind study, adults with active disease were recruited from 80 centers in Australia, Europe, North America, and Taiwan, and were randomized 1:1 to receive placebo or CZP (400 mg at weeks 0, 2, and 4, followed by 200 mg every 2 weeks) in addition to nonbiologic background medication (NBBM). Switching to open-label CZP (or other biologic) or making background medication changes was permitted at any point during the trial, although changes before week 12 were discouraged. The primary end point was the proportion of patients achieving major improvement (MI) (i.e., a ≥2.0-point decrease in the score from baseline or achievement of the lowest possible score [0.6]) in the Ankylosing Spondylitis Disease Activity Score (ASDAS) at week 52.
Results: A total of 317 patients were randomized to receive placebo plus NBBM (n = 158) or CZP plus NBBM (n = 159). ASDAS-MI at week 52 was achieved in 47.2% (75 of 159) of CZP plus NBBM patients, which was significantly greater (P < 0.0001) than the 7.0% (11 of 158) of placebo plus NBBM patients in whom ASDAS-MI was achieved. Of the placebo plus NBBM patients, 60.8% (96 of 158) switched to open-label treatment before week 52 compared to 12.6% (20 of 159) of the CZP plus NBBM patients.
Conclusion: Adding CZP to background medication is superior to adding placebo in patients with active nonradiographic axial SpA. These results indicate that remission in nonradiographic axial SpA treated without biologics occurs infrequently, demonstrating the need for treatment beyond nonbiologic therapy.
Trial registration: ClinicalTrials.gov NCT02552212.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

Similar articles
-
Predictors of long-term clinical response in patients with non-radiographic axial spondyloarthritis receiving certolizumab pegol.Arthritis Res Ther. 2021 Oct 29;23(1):274. doi: 10.1186/s13075-021-02650-4. Arthritis Res Ther. 2021. PMID: 34715908 Free PMC article. Clinical Trial.
-
Impact of Certolizumab Pegol on Patient-Reported Outcomes in Patients With Axial Spondyloarthritis.Arthritis Care Res (Hoboken). 2015 Oct;67(10):1475-80. doi: 10.1002/acr.22594. Arthritis Care Res (Hoboken). 2015. PMID: 25832312 Free PMC article. Clinical Trial.
-
Observed Incidence of Uveitis Following Certolizumab Pegol Treatment in Patients With Axial Spondyloarthritis.Arthritis Care Res (Hoboken). 2016 Jun;68(6):838-44. doi: 10.1002/acr.22848. Arthritis Care Res (Hoboken). 2016. PMID: 26815944 Free PMC article. Clinical Trial.
-
Spotlight on certolizumab pegol in the treatment of axial spondyloarthritis: efficacy, safety and place in therapy.Open Access Rheumatol. 2018 May 7;10:33-41. doi: 10.2147/OARRR.S116654. eCollection 2018. Open Access Rheumatol. 2018. PMID: 29765257 Free PMC article. Review.
-
Certolizumab pegol for treating axial spondyloarthritis.Expert Opin Biol Ther. 2016 Aug;16(8):1059-64. doi: 10.1080/14712598.2016.1205581. Expert Opin Biol Ther. 2016. PMID: 27366922 Review.
Cited by
-
Certolizumab Pegol Treatment in Patients with Axial-Spondyloarthritis-Associated Acute Anterior Uveitis: a Narrative Review.Rheumatol Ther. 2022 Dec;9(6):1481-1497. doi: 10.1007/s40744-022-00486-1. Epub 2022 Sep 30. Rheumatol Ther. 2022. PMID: 36178585 Free PMC article. Review.
-
Low uveitis rates in patients with axial spondyloarthritis treated with bimekizumab: pooled results from phase 2b/3 trials.Ann Rheum Dis. 2024 Nov 14;83(12):1722-1730. doi: 10.1136/ard-2024-225933. Ann Rheum Dis. 2024. PMID: 38977276 Free PMC article.
-
Treatment of Axial Spondyloarthritis: What Does the Future Hold?Curr Rheumatol Rep. 2020 Jul 20;22(9):47. doi: 10.1007/s11926-020-00924-5. Curr Rheumatol Rep. 2020. PMID: 32691259 Free PMC article. Review.
-
Effectiveness and safety of secukinumab in axial spondyloarthritis: a 24-month prospective, multicenter real-life study.Ther Adv Musculoskelet Dis. 2022 Apr 29;14:1759720X221090310. doi: 10.1177/1759720X221090310. eCollection 2022. Ther Adv Musculoskelet Dis. 2022. PMID: 35510168 Free PMC article.
-
Improving the design of RCTs in non-radiographic axial spondyloarthritis.Nat Rev Rheumatol. 2022 Aug;18(8):481-489. doi: 10.1038/s41584-022-00789-1. Epub 2022 May 13. Nat Rev Rheumatol. 2022. PMID: 35562426 Review.
References
-
- Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24‐week results of a double‐blind randomised placebo‐controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. - PMC - PubMed
-
- Sieper J, Kivitz AJ, van Tubergen AM, Deodhar AA, Coteur G, Woltering F, et al. Rapid improvements in patient‐reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis and non‐radiographic axial spondyloarthritis: 24‐week results of a phase 3 double blind randomized placebo‐controlled study [abstract]. Value Health 2013;16 Suppl:A227.
-
- Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. - PubMed
-
- Keat A, Bennett AN, Gaffney K, Marzo‐Ortega H, Sengupta R, Everiss T. Should axial spondyloarthritis without radiographic changes be treated with anti‐TNF agents? Rheumatol Int 2017;37:327–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

