SMAD Signaling Restricts Mucous Cell Differentiation in Human Airway Epithelium
- PMID: 30848657
- PMCID: PMC6839926
- DOI: 10.1165/rcmb.2018-0326OC
SMAD Signaling Restricts Mucous Cell Differentiation in Human Airway Epithelium
Abstract
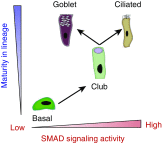
Mucin-secreting goblet cell metaplasia and hyperplasia (GCMH) is a common pathological phenotype in many human respiratory diseases, including asthma, chronic obstructive pulmonary disease, cystic fibrosis, primary ciliary dyskinesia, and infections. A better understanding of how goblet cell quantities or proportions in the airway epithelium are regulated may provide novel therapeutic targets to mitigate GCMH in these devastating diseases. We identify canonical SMAD signaling as the principal pathway restricting goblet cell differentiation in human airway epithelium. Differentiated goblet cells express low levels of phosphorylated SMAD. Accordingly, inhibition of SMAD signaling markedly amplifies GCMH induced by mucous mediators. In contrast, SMAD signaling activation impedes goblet cell generation and accelerates the resolution of preexisting GCMH. SMAD signaling inhibition can override the suppressive effects imposed by a GABAergic receptor inhibitor, suggesting the GABAergic pathway likely operates through inhibition of SMAD signaling in regulating mucous differentiation. Collectively, our data demonstrate that SMAD signaling plays a determining role in mucous cell differentiation, and thus raise the possibility that dysregulation of this pathway contributes to respiratory pathophysiology during airway inflammation and pulmonary diseases. In addition, our study also highlights the potential for SMAD modulation as a therapeutic target in mitigating GCMH.
Keywords: GABAergic pathway; SMAD signaling; airway diseases; goblet cell; human airway epithelium.
Figures
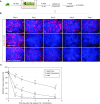
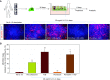
Comment in
-
Escalating Mucus Inhibition to the Top of Our Priorities.Am J Respir Cell Mol Biol. 2019 Sep;61(3):275-276. doi: 10.1165/rcmb.2019-0143ED. Am J Respir Cell Mol Biol. 2019. PMID: 31063695 Free PMC article. No abstract available.
References
-
- Tanabe T, Rubin BK. Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth fa2ctors. Chest. 2016;149:714–720. - PubMed
-
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. - PubMed
-
- Parulekar AD, Kao CC, Diamant Z, Hanania NA. Targeting the interleukin-4 and interleukin-13 pathways in severe asthma: current knowledge and future needs. Curr Opin Pulm Med. 2018;24:50–55. - PubMed
-
- Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Reports. 2015;10:239–252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

