Histological Criteria that Distinguish Human and Mouse Bone Formed Within a Mouse Skeletal Repair Defect
- PMID: 30848692
- PMCID: PMC6542146
- DOI: 10.1369/0022155419836436
Histological Criteria that Distinguish Human and Mouse Bone Formed Within a Mouse Skeletal Repair Defect
Abstract
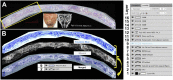
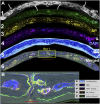
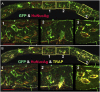
The effectiveness of autologous cell-based skeletal repair continues to be controversial in part because in vitro predictors of in vivo human bone formation by cultured human progenitor cells are not reliable. To assist in the development of in vivo assays of human osteoprogenitor potential, a fluorescence-based histology of nondecalcified mineralized tissue is presented that provides multiple criteria to distinguish human and host osteoblasts, osteocytes, and accumulated bone matrix in a mouse calvarial defect model. These include detection of an ubiquitously expressed red fluorescent protein reporter by the implanted human cells, antibodies specific to human bone sialoprotein and a human nuclear antigen, and expression of a bone/fibroblast restricted green fluorescent protein reporter in the host tissue. Using low passage bone marrow-derived stromal cells, robust human bone matrix formation was obtained. However, a striking feature is the lack of mouse bone marrow investment and osteoclasts within the human bone matrix. This deficiency may account for the accumulation of a disorganized human bone matrix that has not undergone extensive remodeling. These features, which would not be appreciated by traditional decalcified paraffin histology, indicate the human bone matrix is not undergoing active remodeling and thus the full differentiation potential of the implanted human cells within currently used mouse models is not being realized.
Keywords: GFP; RFP; bone cell transplantation; bone matrix; calvarial defect model; cryohistology; epifluorescence imaging; immunostaining; mineralized tissues; primary human MSCs; xenograft.
Conflict of interest statement
Figures


Similar articles
-
Enhanced fatty acid oxidation in osteoprogenitor cells provides protection from high-fat diet induced bone dysfunction.J Bone Miner Res. 2025 Feb 2;40(2):283-298. doi: 10.1093/jbmr/zjae195. J Bone Miner Res. 2025. PMID: 39657629 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Mineralized osteoblast-derived exosomes and 3D-printed ceramic-based scaffolds for enhanced bone healing: A preclinical exploration.Acta Biomater. 2025 Jun 15;200:686-702. doi: 10.1016/j.actbio.2025.05.051. Epub 2025 May 21. Acta Biomater. 2025. PMID: 40409510
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review.Health Technol Assess. 2006 Jul;10(24):iii-iv, ix-136. doi: 10.3310/hta10240. Health Technol Assess. 2006. PMID: 16796930
Cited by
-
Multiplexed tape-stabilized cryohistology of mineralized large animal specimens.J Biol Methods. 2022 Nov 17;9(4):e166. doi: 10.14440/jbm.2022.389. eCollection 2022. J Biol Methods. 2022. PMID: 36992919 Free PMC article.
-
Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice.NPJ Regen Med. 2021 Jun 11;6(1):34. doi: 10.1038/s41536-021-00145-z. NPJ Regen Med. 2021. PMID: 34117259 Free PMC article.
-
Human amniotic fluid stem cells attract osteoprogenitor cells in bone healing.J Cell Physiol. 2020 May;235(5):4643-4654. doi: 10.1002/jcp.29342. Epub 2019 Oct 24. J Cell Physiol. 2020. PMID: 31650536 Free PMC article.
-
Evaluation of an Engineered Hybrid Matrix for Bone Regeneration via Endochondral Ossification.Ann Biomed Eng. 2020 Mar;48(3):992-1005. doi: 10.1007/s10439-019-02279-0. Epub 2019 Apr 29. Ann Biomed Eng. 2020. PMID: 31037444 Free PMC article.
References
-
- Bone Therapeutics: A Regenerative Therapy Company. Available from: www.bonetherapeutics.com
-
- Novadip Biosciences. Available from: http://www.novadip.com
-
- Bone Biologics Corp. Available from: http://bonebiologics.com
-
- Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32(1):160–65. - PubMed
-
- Panetta NJ, Gupta DM, Longaker MT. Bone regeneration and repair. Curr Stem Cell Res Ther. 2010;5(2):122–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

