Gasotransmitters in pregnancy: from conception to uterine involution
- PMID: 30848786
- PMCID: PMC6614580
- DOI: 10.1093/biolre/ioz038
Gasotransmitters in pregnancy: from conception to uterine involution
Abstract
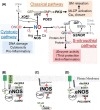
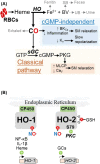
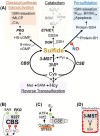
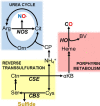
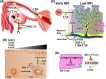
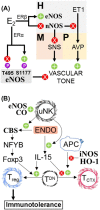
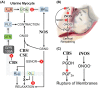
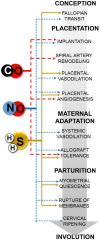
Gasotransmitters are endogenous small gaseous messengers exemplified by nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S or sulfide). Gasotransmitters are implicated in myriad physiologic functions including many aspects of reproduction. Our objective was to comprehensively review basic mechanisms and functions of gasotransmitters during pregnancy from conception to uterine involution and highlight future research opportunities. We searched PubMed and Web of Science databases using combinations of keywords nitric oxide, carbon monoxide, sulfide, placenta, uterus, labor, and pregnancy. We included English language publications on human and animal studies from any date through August 2018 and retained basic and translational articles with relevant original findings. All gasotransmitters activate cGMP signaling. NO and sulfide also covalently modify target protein cysteines. Protein kinases and ion channels transduce gasotransmitter signals, and co-expressed gasotransmitters can be synergistic or antagonistic depending on cell type. Gasotransmitters influence tubal transit, placentation, cervical remodeling, and myometrial contractility. NO, CO, and sulfide dilate resistance vessels, suppress inflammation, and relax myometrium to promote uterine quiescence and normal placentation. Cervical remodeling and rupture of fetal membranes coincide with enhanced oxidation and altered gasotransmitter metabolism. Mechanisms mediating cellular and organismal changes in pregnancy due to gasotransmitters are largely unknown. Altered gasotransmitter signaling has been reported for preeclampsia, intrauterine growth restriction, premature rupture of membranes, and preterm labor. However, in most cases specific molecular changes are not yet characterized. Nonclassical signaling pathways and the crosstalk among gasotransmitters are emerging investigation topics.
Keywords: 3-mercaptosulfurtransferase (3-MST); ATP-gated potassium channel (KATP); calcium-gated potassium channel (BKCa); carbon monoxide (CO); cystathionine-β-synthase (CBS); cystathionine-γ-lyase (CSE); decidua; extravillous trophoblast (EVT); gasotransmitter; heme oxygenase (HO); hydrogen sulfide (H2S); maternal–fetal interface; myometrium; nitric oxide (NO); nitric oxide synthase (NOS); paraventricular nucleus (PVN); parturition; placenta; pregnancy; uterus.
© The Author(s) 2019. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures
References
-
- Friebe A, Koesling D. Mechanism of YC-1-induced activation of soluble guanylyl cyclase. Mol Pharmacol 1998; 53(1):123–127. - PubMed
-
- Lewicki JA, Brandwein HJ, Mittal CK, Arnold WP, Murad F. Properties of purified soluble guanylate cyclase activated by nitric oxide and sodium nitroprusside. J Cyclic Nucleotide Res 1982; 8(1):17–25. - PubMed
-
- Kulandavelu S, Whiteley KJ, Qu D, Mu J, Bainbridge SA, Adamson SL. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension 2012; 60(1):231–238. - PubMed
-
- Nuño-Ayala M, Guillén N, Arnal C, Lou-Bonafonte JM, de Martino A, García-de-Jalón JA, Gascón S, Osaba L, Osada J, Navarro MA. Cystathionine β-synthase deficiency causes infertility by impairing decidualization and gene expression networks in uterus implantation sites. Physiol Genomics 2012; 44(14):702–716. - PubMed

