Site-Specific 1D and 2D IR Spectroscopy to Characterize the Conformations and Dynamics of Protein Molecular Recognition
- PMID: 30848912
- PMCID: PMC6547822
- DOI: 10.1021/acs.jpcb.9b00969
Site-Specific 1D and 2D IR Spectroscopy to Characterize the Conformations and Dynamics of Protein Molecular Recognition
Abstract
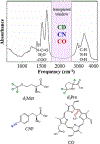
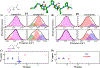
Proteins exist as ensembles of interconverting states on a complex energy landscape. A complete, molecular-level understanding of their function requires knowledge of the populated states and thus the experimental tools to characterize them. Infrared (IR) spectroscopy has an inherently fast time scale that can capture all states and their dynamics with, in principle, bond-specific spatial resolution, and 2D IR methods that provide richer information are becoming more routine. Although application of IR spectroscopy for investigation of proteins is challenged by spectral congestion, the issue can be overcome by site-specific introduction of amino acid side chains that have IR probe groups with frequency-resolved absorptions, which furthermore enables selective characterization of different locations in proteins. Here, we briefly introduce the biophysical methods and summarize the current progress toward the study of proteins. We then describe our efforts to apply site-specific 1D and 2D IR spectroscopy toward elucidation of protein conformations and dynamics to investigate their involvement in protein molecular recognition, in particular mediated by dynamic complexes: plastocyanin and its binding partner cytochrome f, cytochrome P450s and substrates or redox partners, and Src homology 3 domains and proline-rich peptide motifs. We highlight the advantages of frequency-resolved probes to characterize specific, local sites in proteins and uncover variation among different locations, as well as the advantage of the fast time scale of IR spectroscopy to detect rapidly interconverting states. In addition, we illustrate the greater insight provided by 2D methods and discuss potential routes for further advancement of the field of biomolecular 2D IR spectroscopy.
Figures



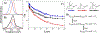


References
-
- Frauenfelder H; Sligar SG; Wolynes PG The Energy Landscapes and Motions of Proteins. Science 1991, 254, 1598–1603. - PubMed
-
- Boxer SG Stark Realities. J. Phys. Chem. B 2009, 113, 2972–2983. - PubMed
-
- Blasiak B; Londergan CH; Webb LJ; Cho M Vibrational Probes: From Small Molecule Solvatochromism Theory and Experiments to Applications in Complex Systems. Acc. Chem. Res. 2017, 50, 968–976. - PubMed
-
- Mukamel S Principles of Nonlinear Optics and Spectroscopy; Oxford University Press: New York, 1995.
-
- Cho M Two-Dimensional Optical Spectroscopy; CRC Press: Boca Raton, FL, 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

