Desialylation of platelets induced by Von Willebrand Factor is a novel mechanism of platelet clearance in dengue
- PMID: 30849118
- PMCID: PMC6426266
- DOI: 10.1371/journal.ppat.1007500
Desialylation of platelets induced by Von Willebrand Factor is a novel mechanism of platelet clearance in dengue
Abstract
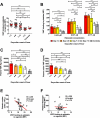
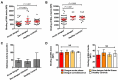
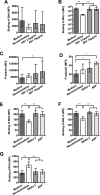
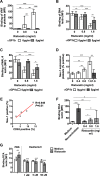
Thrombocytopenia and platelet dysfunction are commonly observed in patients with dengue virus (DENV) infection and may contribute to complications such as bleeding and plasma leakage. The etiology of dengue-associated thrombocytopenia is multifactorial and includes increased platelet clearance. The binding of the coagulation protein von Willebrand factor (VWF) to the platelet membrane and removal of sialic acid (desialylation) are two well-known mechanisms of platelet clearance, but whether these conditions also contribute to thrombocytopenia in dengue infection is unknown. In two observational cohort studies in Bandung and Jepara, Indonesia, we show that adult patients with dengue not only had higher plasma concentrations of plasma VWF antigen and active VWF, but that circulating platelets had also bound more VWF to their membrane. The amount of platelet-VWF binding correlated well with platelet count. Furthermore, sialic acid levels in dengue patients were significantly reduced as assessed by the binding of Sambucus nigra lectin (SNA) and Maackia amurensis lectin II (MAL-II) to platelets. Sialic acid on the platelet membrane is neuraminidase-labile, but dengue virus has no known neuraminidase activity. Indeed, no detectable activity of neuraminidase was present in plasma of dengue patients and no desialylation was found of plasma transferrin. Platelet sialylation was also not altered by in vitro exposure of platelets to DENV nonstructural protein 1 or cultured DENV. In contrast, induction of binding of VWF to glycoprotein 1b on platelets using the VWF-activating protein ristocetin resulted in the removal of platelet sialic acid by translocation of platelet neuraminidase to the platelet surface. The neuraminidase inhibitor oseltamivir reduced VWF-induced platelet desialylation. Our data demonstrate that excessive binding of VWF to platelets in dengue results in neuraminidase-mediated platelet desialylation and platelet clearance. Oseltamivir might be a novel treatment option for severe thrombocytopenia in dengue infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Type IIB von Willebrand factor with normal sialic acid content induces platelet aggregation in the absence of ristocetin. Role of platelet activation, fibrinogen, and two distinct membrane receptors.J Clin Invest. 1987 Aug;80(2):475-82. doi: 10.1172/JCI113095. J Clin Invest. 1987. PMID: 3038958 Free PMC article.
-
Effect of carbohydrate modifications of factor VIII/von Willebrand factor on binding to platelets.Thromb Haemost. 1985 Jun 24;53(3):390-5. Thromb Haemost. 1985. PMID: 2864750
-
von Willebrand factor binds to platelets and induces aggregation in platelet-type but not type IIB von Willebrand disease.J Clin Invest. 1983 Nov;72(5):1532-42. doi: 10.1172/JCI111112. J Clin Invest. 1983. PMID: 6415113 Free PMC article.
-
Platelets and von Willebrand factor.Transfus Apher Sci. 2003 Jun;28(3):269-77. doi: 10.1016/S1473-0502(03)00046-6. Transfus Apher Sci. 2003. PMID: 12725954 Review.
-
Platelet von Willebrand factor--structure, function and biological importance.Br J Haematol. 2010 Mar;148(6):834-43. doi: 10.1111/j.1365-2141.2009.08052.x. Epub 2010 Jan 11. Br J Haematol. 2010. PMID: 20067560 Review.
Cited by
-
Platelet Activation Mechanisms and Consequences of Immune Thrombocytopenia.Cells. 2021 Dec 1;10(12):3386. doi: 10.3390/cells10123386. Cells. 2021. PMID: 34943895 Free PMC article. Review.
-
Structure-function of platelet glycoprotein Ib-IX.J Thromb Haemost. 2020 Dec;18(12):3131-3141. doi: 10.1111/jth.15035. Epub 2020 Aug 24. J Thromb Haemost. 2020. PMID: 32735697 Free PMC article. Review.
-
Multifaceted role of glycosylation in transfusion medicine, platelets, and red blood cells.J Thromb Haemost. 2020 Jul;18(7):1535-1547. doi: 10.1111/jth.14874. Epub 2020 May 28. J Thromb Haemost. 2020. PMID: 32350996 Free PMC article. Review.
-
Molecular Determinants of Tissue Specificity of Flavivirus Nonstructural Protein 1 Interaction with Endothelial Cells.J Virol. 2022 Oct 12;96(19):e0066122. doi: 10.1128/jvi.00661-22. Epub 2022 Sep 15. J Virol. 2022. PMID: 36106873 Free PMC article.
-
Increased von Willebrand Factor Platelet-Binding Capacity Is Related to Poor Prognosis in COVID-19 Patients.Thromb Haemost. 2023 Jan;123(1):118-122. doi: 10.1055/a-1962-5447. Epub 2022 Oct 17. Thromb Haemost. 2023. PMID: 36252812 Free PMC article. No abstract available.
References
-
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control: World Health Organization; 2009. - PubMed
-
- Tomashek KM, Lorenzi OD, Andujar-Perez DA, Torres-Velasquez BC, Hunsperger EA, Munoz-Jordan JL, et al. Clinical and epidemiologic characteristics of dengue and other etiologic agents among patients with acute febrile illness, Puerto Rico, 2012–2015. PLoS Negl Trop Dis. 2017;11(9):e0005859 10.1371/journal.pntd.0005859 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

