Immunogenicity and Safety of a Measles-Mumps-Rubella Vaccine Administered as a First Dose to Children Aged 12 to 15 Months: A Phase III, Randomized, Noninferiority, Lot-to-Lot Consistency Study
- PMID: 30849175
- PMCID: PMC7192400
- DOI: 10.1093/jpids/piz010
Immunogenicity and Safety of a Measles-Mumps-Rubella Vaccine Administered as a First Dose to Children Aged 12 to 15 Months: A Phase III, Randomized, Noninferiority, Lot-to-Lot Consistency Study
Abstract
Background: MMR II (M-M-R II [Merck & Co, Inc.]) is currently the only measles, mumps, and rubella (MMR) vaccine licensed in the United States. A second MMR vaccine would mitigate the potential risk of vaccine supply shortage or delay. In this study, we assessed the immunogenicity and safety of another MMR vaccine (MMR-RIT [Priorix, GlaxoSmithKline]) compared with those of the MMR II in 12- to 15-month-old children who received it as a first dose.
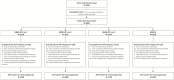
Methods: In this phase III, observer-blinded, noninferiority, lot-to-lot consistency clinical trial (ClinicalTrials.gov identifier NCT01702428), 5003 healthy children were randomly assigned to receive 1 dose of MMR-RIT (1 of 3 production lots) or MMR II along with other age-recommended routine vaccines. We evaluated the immunogenicity of all vaccines in terms of antibody concentrations (by using an enzyme-linked immunosorbent assay or electrochemiluminescence assay) and/or seroresponse rates 43 days after vaccination. We also assessed the reactogenicity and safety of the vaccines.
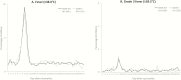
Results: Immunoresponses after vaccination with MMR-RIT were robust and noninferior to those after vaccination with the MMR II. Immunogenicity of the 3 production lots of MMR-RIT was consistent; more than 97% of the children had a seroresponse to MMR components. The coadministered vaccines elicited similar immunoresponses in the MMR-RIT and MMR II groups. Both MMR vaccines resulted in comparable reactogenicity profiles, and no safety concerns were detected.
Conclusions: If licensed, the MMR-RIT could provide a valid option for the prevention of measles, mumps, and rubella in children in the United States and would reduce potential risks of a vaccine shortage.
Keywords: vaccine; immunogenicity; safety.
© The Author(s) 2019. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society.
Figures

References
-
- Food and Drug Administration. M-M-R II: package insert. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/UCM123789.pdf. Accessed April 28, 2018.
-
- Centers for Disease Control and Prevention. Measles—United States, 2000. MMWR Morb Mortal Wkly Rep 2002; 51:120–3. - PubMed
-
- Centers for Disease Control and Prevention. Chapter 20: rubella. In: Hamborsky J, Kroger A, Wolfe S, eds. The Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th ed. Washington DC: Public Health Foundation; 2015: pp 325–40.
-
- Centers for Disease Control and Prevention. Mumps cases and outbreaks. Available at: https://www.cdc.gov/mumps/outbreaks.html. Accessed March 13, 2018.
-
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Available at: https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 13, 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

