Broad-spectrum coronavirus antiviral drug discovery
- PMID: 30849247
- PMCID: PMC7103675
- DOI: 10.1080/17460441.2019.1581171
Broad-spectrum coronavirus antiviral drug discovery
Abstract
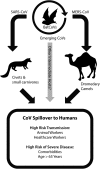
The highly pathogenic coronaviruses severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are lethal zoonotic viruses that have emerged into human populations these past 15 years. These coronaviruses are associated with novel respiratory syndromes that spread from person-to-person via close contact, resulting in high morbidity and mortality caused by the progression to Acute Respiratory Distress Syndrome (ARDS). Areas covered: The risks of re-emergence of SARS-CoV from bat reservoir hosts, the persistence of MERS-CoV circulation, and the potential for future emergence of novel coronaviruses indicate antiviral drug discovery will require activity against multiple coronaviruses. In this review, approaches that antagonize viral nonstructural proteins, neutralize structural proteins, or modulate essential host elements of viral infection with varying levels of efficacy in models of highly pathogenic coronavirus disease are discussed. Expert opinion: Treatment of SARS and MERS in outbreak settings has focused on therapeutics with general antiviral activity and good safety profiles rather than efficacy data provided by cellular, rodent, or nonhuman primate models of highly pathogenic coronavirus infection. Based on lessons learned from SARS and MERS outbreaks, lack of drugs capable of pan-coronavirus antiviral activity increases the vulnerability of public health systems to a highly pathogenic coronavirus pandemic.
Keywords: ARDS; Antiviral; MERS; MERS-CoV; Middle East respiratory syndrome; SARS; SARS-CoV; acute respiratory distress syndrome; bat; broad-spectrum; camel; civet; coronavirus; emerging virus; highly pathogenic virus; human cases; in vitro model; interferon; lopinavir; pneumonia; primate model; respiratory; ribavirin; rodent model; severe acute respiratory syndrome; therapeutic; zoonosis; zoonotic.
Figures


Comment in
-
Coronavirus 2019-nCoV: Is the genie already out of the bottle?Travel Med Infect Dis. 2020 May-Jun;35:101577. doi: 10.1016/j.tmaid.2020.101577. Epub 2020 Feb 7. Travel Med Infect Dis. 2020. PMID: 32044388 Free PMC article. No abstract available.
References
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. - PubMed
-
- Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. - PubMed
-
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. - PubMed
-
- Lew TWK, Kwek T-K, Tai D, et al. Acute respiratory distress syndrome in critically Ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. Available from: http://jama.ama-assn.org/cgi/content/abstract/290/3/374 - PubMed
-
- WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. [cited 2019 January25] Available from: http://www.who.int/csr/sars/country/table2004_04_21/en/index.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
