Inhibition of Cdc42-intersectin interaction by small molecule ZCL367 impedes cancer cell cycle progression, proliferation, migration, and tumor growth
- PMID: 30849276
- PMCID: PMC6606017
- DOI: 10.1080/15384047.2018.1564559
Inhibition of Cdc42-intersectin interaction by small molecule ZCL367 impedes cancer cell cycle progression, proliferation, migration, and tumor growth
Abstract
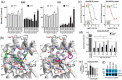
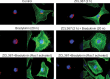
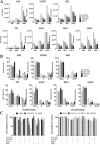
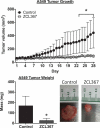
Cdc42 is a member of the Rho family of small GTPases that are at the crossroads of major oncogenic signaling pathways involved in both lung and prostate cancers. However, the therapeutic potential of Cdc42 regulation is still unclear due to the lack of pharmacological tools. Herein, we report that ZCL367 is a bona fide Cdc42 inhibitor that suppressed cancer development and ZCL278 can act as a partial Cdc42 agonist. In lung cancer cell lines with varying EGFR and Ras mutations as well as both androgen-independent and androgen-dependent prostate cancer cell lines, ZCL367 impeded cell cycle progression, reduced proliferation, and suppressed migration. ZCL367 decreased Cdc42-intersectin interactions and reduced Cdc42-mediated filopodia formation. ZCL367 showed increased potency and selectivity for Cdc42 when compared to Rac1 and RhoA. ZCL367 reduced A549 tumorigenesis in a xenograft mouse model. Altogether, ZCL367 is a selective Cdc42 inhibitor and an excellent candidate for lead compound optimization for further anticancer studies.
Keywords: Cdc42; Rho GTPase; drug development; intersectin; lung cancer; prostate cancer.
Figures

References
-
- Rihet S, Vielh P, Camonis J, Goud B, Chevillard S, de Gunzburg J. Mutation status of genes encoding RhoA, Rac1, and Cdc42 GTPases in a panel of invasive human colorectal and breast tumors. J Cancer Res Clin Oncol. 2001;127:733–738. - PubMed
-
- Knyazev YP, Cheburkin YV, Spikermann K, Peter S, Jenster G, Bangma KH, Karelin MI, Shkolnik MI, Urbanskii AI, Evtushenko VI. The cDNA microarray profiling of protein kinases and phosphatases: molecular portrait of human prostate carcinomas. Mol Biol [Internet] 2003;37:89–101. http://link.springer.com/article/10.1023/A:1022341015018 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
