A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery
- PMID: 30849470
- PMCID: PMC6562166
- DOI: 10.1016/j.ijpharm.2019.03.004
A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery
Abstract
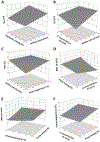
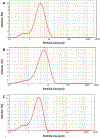
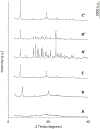
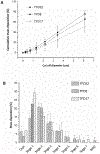
The present study evaluates the effect of L-leucine concentration and operating parameters of a laboratory spray dryer on characteristics of trehalose dry powders, with the goal of optimizing production of these powders for inhaled drug delivery. Trehalose/L-leucine mixtures were spray dried from aqueous solution using a laboratory spray dryer. A factorial design of experiment (DoE) was undertaken and process parameters adjusted were: inlet temperature, gas flow rate, feed solution flow rate (pump setting), aspiration setting and L-leucine concentration. Resulting powders were characterised in terms of particle size, yield, residual moisture content, and glass transition temperature. Particle size was mainly influenced by gas flow rate, whereas product yield and residual moisture content were found to be primarily affected by inlet temperature and spray solution feed rate respectively. Interactions between a number of different process parameters were elucidated, as were relationships between different responses. The leucine mass ratio influenced the physical stability of powders against environmental humidity, and a high leucine concentration (30% w/w) protected amorphous trehalose from moisture induced crystallization. High weight ratio of leucine in the formulation, however, negatively impacted the aerosol performance. Thus, in terms of L-leucine inclusion in a formulation designed for pulmonary delivery, a balance needs to be found between physical stability and deposition characteristics.
Keywords: DPI; Design of experiments; Dry powder for inhalation; Leucine; Pulmonary formulation; Spray drying; Trehalose.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Understanding the impact of L-leucine on physical stability and aerosolization of spray-dried protein powder formulations containing trehalose.Int J Pharm. 2025 May 30;677:125636. doi: 10.1016/j.ijpharm.2025.125636. Epub 2025 Apr 22. Int J Pharm. 2025. PMID: 40273961
-
The use of hydrophobic amino acids in protecting spray dried trehalose formulations against moisture-induced changes.Eur J Pharm Biopharm. 2019 Nov;144:139-153. doi: 10.1016/j.ejpb.2019.09.014. Epub 2019 Sep 16. Eur J Pharm Biopharm. 2019. PMID: 31536784 Free PMC article.
-
Optimisation of spray drying process conditions for sugar nanoporous microparticles (NPMPs) intended for inhalation.Int J Pharm. 2011 Dec 12;421(1):99-109. doi: 10.1016/j.ijpharm.2011.09.021. Epub 2011 Sep 21. Int J Pharm. 2011. PMID: 21963473
-
Spray-Dried PulmoSphere™ Formulations for Inhalation Comprising Crystalline Drug Particles.AAPS PharmSciTech. 2019 Feb 7;20(3):103. doi: 10.1208/s12249-018-1280-0. AAPS PharmSciTech. 2019. PMID: 30734187 Review.
-
Bacteriophage Encapsulation Using Spray Drying for Phage Therapy.Curr Issues Mol Biol. 2021;40:303-316. doi: 10.21775/cimb.040.303. Epub 2020 Jul 17. Curr Issues Mol Biol. 2021. PMID: 32678066 Review.
Cited by
-
Hierarchy of the Components in Spray-Dried, Protein-Excipient Particles Using DNP-Enhanced NMR Spectroscopy.Mol Pharm. 2023 Nov 6;20(11):5682-5689. doi: 10.1021/acs.molpharmaceut.3c00539. Epub 2023 Oct 2. Mol Pharm. 2023. PMID: 37782000 Free PMC article.
-
Lipid-coated bismuth nanoflower as the thermos-radio sensiti for therapy of lung metastatic breast cancer: Preparation, optimisation, and characterisation.IET Nanobiotechnol. 2022 Dec;16(9):305-315. doi: 10.1049/nbt2.12097. Epub 2022 Aug 29. IET Nanobiotechnol. 2022. PMID: 36036543 Free PMC article.
-
Advancements in Particle Engineering for Inhalation Delivery of Small Molecules and Biotherapeutics.Pharm Res. 2022 Dec;39(12):3047-3061. doi: 10.1007/s11095-022-03363-2. Epub 2022 Sep 7. Pharm Res. 2022. PMID: 36071354 Free PMC article. Review.
-
Formulation and In Vitro and In Silico Characterization of "Nano-in-Micro" Dry Powder Inhalers Containing Meloxicam.Pharmaceutics. 2021 Feb 3;13(2):211. doi: 10.3390/pharmaceutics13020211. Pharmaceutics. 2021. PMID: 33546452 Free PMC article.
-
Design of Experiment (DoE) Approach for Developing Inhalable PLGA Microparticles Loaded with Clofazimine for Tuberculosis Treatment.Pharmaceuticals (Basel). 2024 Jun 7;17(6):754. doi: 10.3390/ph17060754. Pharmaceuticals (Basel). 2024. PMID: 38931422 Free PMC article.
References
-
- Amaro MI, Tajber L, Corrigan OI, Healy AM, 2011. Optimisation of spray drying process conditions for sugar nanoporous microparticles (NPMPs) intended for inhalation. International Journal of Pharmaceutics 421, 99–109. - PubMed
-
- Amaro MI, Tajber L, Corrigan OI, Healy AM, 2015a. Co-spray dried carbohydrate microparticles: crystallisation delay/inhibition and improved aerosolization characteristics through the incorporation of hydroxypropyl-beta-cyclodextrin with amorphous raffinose or trehalose. Pharm Res 32, 180–195. - PubMed
-
- Amaro MI, Tewes F, Gobbo O, Tajber L, Corrigan OI, Ehrhardt C, Healy AM, 2015b. Formulation, stability and pharmacokinetics of sugar-based salmon calcitonin-loaded nanoporous/nanoparticulate microparticles (NPMPs) for inhalation. International Journal of Pharmaceutics 483, 6–18. - PubMed
-
- Aquino RP, Prota L, Auriemma G, Santoro A, Mencherini T, Colombo G, Russo P, 2012. Dry powder inhalers of gentamicin and leucine: formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. International Journal of Pharmaceutics 426, 100–107. - PubMed
-
- Billon A, Bataille B, Cassanas G, Jacob M, 2000. Development of spray-dried acetaminophen microparticles using experimental designs. International Journal of Pharmaceutics 203, 159–168. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

