Cysteine-rich intestinal protein 1 suppresses apoptosis and chemosensitivity to 5-fluorouracil in colorectal cancer through ubiquitin-mediated Fas degradation
- PMID: 30850009
- PMCID: PMC6408822
- DOI: 10.1186/s13046-019-1117-z
Cysteine-rich intestinal protein 1 suppresses apoptosis and chemosensitivity to 5-fluorouracil in colorectal cancer through ubiquitin-mediated Fas degradation
Abstract
Background: Cysteine-rich intestinal protein 1 (CRIP1) is highly expressed in human intestine and aberrantly expressed in several types of tumor. However, studies on CRIP1 are limited and its role on tumor development and progression remains controversial and elusive.
Methods: Immunohistochemistry was performed to evaluate the expression of CRIP1 in paired normal and colorectal tumor specimens, as well as colorectal cell lines. Functional assays, such as CCK8, TUNEL assay and in vivo tumor growth assay, were used to detect the proliferation, apoptosis and response to 5-FU of CRIP1. Western blot was used to analyze Fas-mediated pathway induced by CRIP1. Rescue experiments were performed to evaluate the essential role of CRIP1 for Fas-mediated apoptosis.
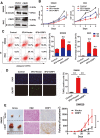
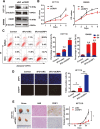
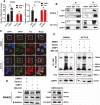
Results: We demonstrated that CRIP1 is overexpressed in CRC tissues compared with adjacent normal mucosa. CRIP1 could dramatically recover the 5-Fluorouracil (5-FU) inhibited CRC cell proliferation in vitro and stimulate the tumor formation of CRC in vivo, probably through inhibiting CRC cell apoptosis. Moreover, CRIP1 also dramatically recovered the 5-Fluorouracil (5-FU) induced tumor cell apoptosis in vitro. Further study demonstrated that CRIP1 down-regulated the expression of Fas protein and proteins related to Fas-mediated apoptosis. CRIP1 could interact with Fas protein and stimulate its ubiquitination and degradation. In addition, a negative correlation was detected between the expression of CRIP1 and Fas protein in most of the clinical human CRC samples.
Conclusion: The current research reveals a vital role of CRIP1 in CRC progression, which provide a novel target for clinical drug resistance of colorectal cancer and undoubtedly contributing to the therapeutic strategies in CRC.
Keywords: Apoptosis; Chemoresistant; Colorectal cancer; Cysteine-rich intestinal protein 1; FAS.
Conflict of interest statement
Ethics approval
All experiments involving patients are endorsed by the Ethics Committee of Southern Medical University and complied with the Declaration of Helsinki. No informed consent was required because data were going to be analyzed anonymously. All animal experiments involved ethical and humane treatment under a license from the Guangdong Provincial Bureau of Science.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

